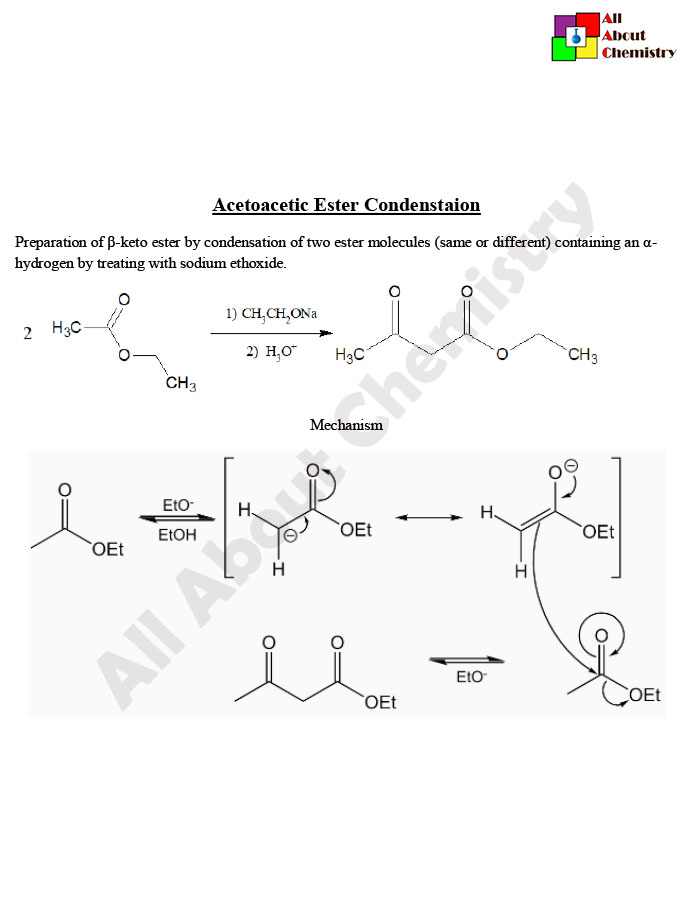
The Acetoacetic Ester Condensation, also known as the Claisen condensation of ethyl acetoacetate, is a classic organic reaction used to synthesize β-keto esters. It involves the reaction of ethyl acetoacetate with a strong base, typically an alkoxide ion, to form a β-keto ester.
Here’s the general reaction:
Ethyl acetoacetate+Base→β-keto ester+Alcohol
The reaction proceeds via a nucleophilic addition-elimination mechanism. The ethoxide ion acts as a strong base, abstracting a proton from the α-carbon of ethyl acetoacetate, resulting in the formation of an enolate ion. This enolate ion then undergoes nucleophilic attack on another molecule of ethyl acetoacetate, leading to the formation of a β-keto ester intermediate. Finally, the β-keto ester intermediate is protonated by the solvent (or another molecule of ethyl acetoacetate), yielding the desired product along with ethanol as a byproduct.
The mechanism of the Acetoacetic Ester Condensation, also known as the Claisen condensation of ethyl acetoacetate, involves several steps and is typically carried out under basic conditions. Here’s a detailed description of the mechanism:
- Formation of Enolate Ion: In the presence of a strong base, such as sodium ethoxide (NaOEt), ethyl acetoacetate undergoes deprotonation at the α-carbon adjacent to both carbonyl groups. This deprotonation occurs because the α-hydrogens are relatively acidic due to the presence of the neighboring carbonyl groups. The base abstracts a proton from the α-carbon, leading to the formation of the enolate ion:Ethyl acetoacetate→Enolate ion+Ethoxide ionEthyl acetoacetate→Enolate ion+Ethoxide ion
- Nucleophilic Addition: The enolate ion acts as a nucleophile and attacks the carbonyl carbon of another molecule of ethyl acetoacetate. This step results in the formation of a tetrahedral intermediate.
- Formation of β-Keto Ester Intermediate: Collapse of the tetrahedral intermediate leads to the formation of a β-keto ester intermediate. The oxygen atom that was part of the enolate group becomes doubly bonded to the carbonyl carbon, resulting in the formation of the β-keto ester.
- Proton Transfer: In the final step, a proton is transferred from the solvent or another molecule of ethyl acetoacetate to the oxygen atom of the β-keto ester intermediate, leading to the formation of the final product. This protonation regenerates the base catalyst, closing the catalytic cycle.
The Acetoacetic Ester Condensation, also known as the Claisen condensation of ethyl acetoacetate, finds numerous applications in organic synthesis due to its ability to produce β-keto esters, which are versatile intermediates for the synthesis of various organic compounds. Some key applications include:
- Synthesis of β-Diketones: The β-keto esters obtained from the Acetoacetic Ester Condensation can be further reacted to yield β-diketones. These β-diketones are valuable building blocks in organic synthesis and are utilized in the synthesis of complex molecules such as natural products and pharmaceuticals.
- Formation of β-Keto Acids: Hydrolysis of the β-keto esters obtained from the condensation reaction leads to the formation of β-keto acids. These β-keto acids are important intermediates in organic synthesis and serve as precursors for the synthesis of various pharmaceuticals, agrochemicals, and fine chemicals.
- Synthesis of α,β-Unsaturated Carbonyl Compounds: The β-keto esters obtained from the condensation reaction can undergo decarboxylation followed by elimination to yield α,β-unsaturated carbonyl compounds. These compounds are widely used in the synthesis of natural products, pharmaceuticals, and functional materials.
- Preparation of Substituted Acetic Acid Derivatives: The β-keto esters obtained from the condensation reaction can be further functionalized to yield substituted acetic acid derivatives. These derivatives find applications in medicinal chemistry, agrochemicals, and materials science.
- Construction of Carbon Skeletons: The Acetoacetic Ester Condensation is often employed in the synthesis of complex molecules to construct carbon-carbon bonds and introduce functional groups at specific positions in the molecule. It serves as a versatile tool for the synthesis of diverse organic compounds.
- Cascade Reactions: The β-keto esters obtained from the condensation reaction can participate in cascade reactions, where multiple bond-forming events occur in a single transformation. Cascade reactions enable the efficient synthesis of complex molecules from simple starting materials.
The Acetoacetic Ester Condensation is widely used in organic synthesis for the preparation of β-keto esters, which serve as important intermediates for the synthesis of various compounds, including pharmaceuticals, natural products, and fine chemicals. Additionally, the reaction is particularly useful in the construction of carbon-carbon bonds, making it a versatile tool in organic chemistry.
Overall, the Acetoacetic Ester Condensation is a valuable synthetic method in organic chemistry, offering a straightforward route to β-keto esters and enabling the synthesis of a wide range of organic compounds with diverse applications in medicinal chemistry, materials science, and other fields.








