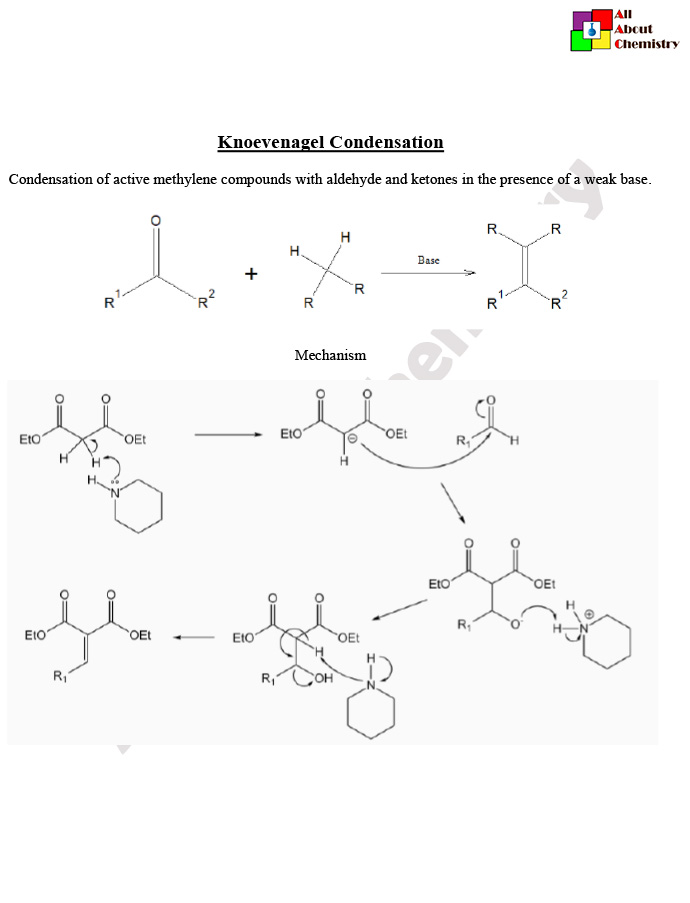
The Knoevenagel condensation is a carbon-carbon bond-forming reaction that involves the condensation of an aldehyde or ketone with a compound containing an active methylene group (a carbon atom bound to two hydrogen atoms and a carbonyl group). The reaction is typically catalyzed by a base, such as a secondary amine or alkoxide.
The general reaction scheme for the Knoevenagel condensation is as follows:
RCHO or RCOR’+CH2(R”)C(O)Y→RCH=C(R’)Y+H2O
Where:
- R, R’, and R” are organic substituents,
- Y represents an electron-withdrawing group, typically a carbonyl group or a nitrile.
In the Knoevenagel condensation, the hydrogen atom on the carbon adjacent to the carbonyl group (active methylene group) is acidic due to the electron-withdrawing nature of the carbonyl group. This makes it susceptible to deprotonation by a base, resulting in the formation of an enolate ion. The enolate ion then undergoes nucleophilic attack on the carbonyl carbon of the aldehyde or ketone, leading to the formation of a β-hydroxy aldehyde or ketone intermediate. This intermediate subsequently loses a molecule of water to afford the final product, which is an α,β-unsaturated carbonyl compound.
The mechanism of the Knoevenagel condensation involves several steps. Here’s a detailed explanation:
- Formation of Enolate Ion: The reaction begins with the deprotonation of the α-carbon adjacent to the carbonyl group (active methylene group) by a base. The base abstracts the relatively acidic hydrogen atom, forming an enolate ion. The base used is typically a secondary amine or alkoxide.RCH(O)C(Y)H+Base→RCH(O)C(Y)−+H2O
- Nucleophilic Addition: The enolate ion acts as a nucleophile and attacks the electrophilic carbon of the carbonyl group of the aldehyde or ketone. This results in the formation of a tetrahedral intermediate.RCH(O)C(Y)−+R′(O)C(Y)→RCH(O)C(Y)C(R’)(O)Y−
- Proton Transfer and Elimination: The tetrahedral intermediate undergoes a proton transfer, leading to the formation of a β-hydroxy aldehyde or ketone. This intermediate subsequently loses a molecule of water through an elimination reaction, resulting in the formation of the final product, which is an α,β-unsaturated carbonyl compound. RCH(O)C(Y)C(R’)(O)Y− = RCH(O)C(R’)=C(O)Y+H2O−
- Tautomerization: In some cases, tautomerization of the β-hydroxy aldehyde or ketone intermediate may occur to form the corresponding enol tautomer, which can then undergo keto-enol tautomerization to yield the final α,β-unsaturated carbonyl compound.
Overall, the Knoevenagel condensation involves the formation of a new carbon-carbon bond between the carbonyl carbon of the aldehyde or ketone and the α-carbon of the compound containing the active methylene group, leading to the formation of an α,β-unsaturated carbonyl compound. The reaction is catalyzed by a base and proceeds via nucleophilic addition-elimination mechanisms.
The Knoevenagel condensation finds broad applications in organic synthesis due to its ability to form α,β-unsaturated carbonyl compounds, which serve as versatile intermediates for the construction of complex molecules. Some of the key applications of the Knoevenagel condensation include:
- Synthesis of Pharmaceuticals: The α,β-unsaturated carbonyl compounds synthesized via the Knoevenagel condensation serve as key intermediates in the synthesis of various pharmaceuticals. These compounds can be further functionalized to introduce specific pharmacophores, allowing for the construction of biologically active molecules.
- Natural Product Synthesis: Many natural products contain α,β-unsaturated carbonyl functionalities in their structures. The Knoevenagel condensation provides a valuable tool for the synthesis of such natural products or their analogs. By strategically incorporating α,β-unsaturated carbonyl moieties, chemists can mimic the structures of natural products and study their biological activities.
- Fine Chemicals Synthesis: The Knoevenagel condensation is widely used in the synthesis of fine chemicals, such as flavors, fragrances, and dyes. The ability to efficiently access α,β-unsaturated carbonyl compounds allows for the preparation of a diverse array of compounds with desirable sensory or color properties.
- Materials Science: α,β-Unsaturated carbonyl compounds synthesized via the Knoevenagel condensation can be utilized in the preparation of materials with specific properties. For example, they can serve as building blocks for the synthesis of polymers, crosslinking agents, or functionalized surfaces.
- Catalysis: α,β-Unsaturated carbonyl compounds obtained from the Knoevenagel condensation can act as versatile substrates in various catalytic transformations. These compounds can participate in a range of reactions, including Michael additions, Robinson annulations, and Diels-Alder reactions, thereby enabling the synthesis of structurally complex molecules under mild conditions.
- Medicinal Chemistry: In drug discovery and development, α,β-unsaturated carbonyl compounds are of particular interest due to their potential as Michael acceptors. They can react with nucleophilic residues in proteins, leading to irreversible inhibition of enzyme activity. This reactivity has been exploited in the design of covalent drugs targeting specific disease-related proteins.
Overall, the Knoevenagel condensation is a powerful synthetic tool that finds wide-ranging applications in both academic research and industrial settings, contributing to the synthesis of diverse organic molecules with important biological, pharmaceutical, and materials science applications.










