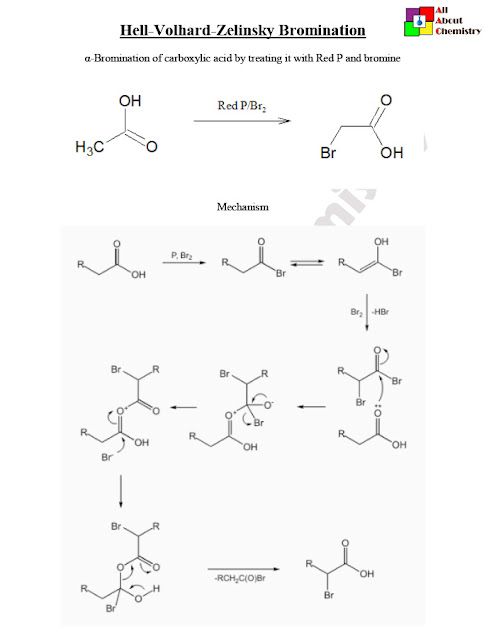
The Hell-Volhard-Zelinsky (HVZ) bromination reaction is a method used to selectively brominate α-carbon atoms of carboxylic acids, producing α-bromo carboxylic acids. This reaction is particularly useful because it allows for the introduction of bromine at the alpha position of a carboxylic acid without affecting other functional groups.
Overall, the reaction can be represented as:
RCOOH+Br2+PBr3→RCOBr+HBr+PBr2
RCOBr+RCOOH→RCOOR’+HBr
Where R and R’ represent alkyl or aryl groups.
The HVZ bromination reaction is widely used in organic synthesis for the preparation of α-bromo carboxylic acids, which can serve as versatile intermediates for various organic transformations. It’s worth noting that while this reaction is commonly associated with bromination, it can also be adapted for chlorination by substituting bromine with chlorine sources.
The Hell-Volhard-Zelinsky (HVZ) bromination mechanism involves two key steps: the formation of an acyl bromide intermediate and the subsequent reaction of this intermediate with the carboxylic acid. Let’s break down the mechanism step by step:
- Formation of the Acyl Bromide Intermediate:
- The reaction begins with the carboxylic acid (RCOOH) and bromine (Br2) in the presence of a catalytic amount of phosphorus tribromide (PBr3).Phosphorus tribromide (PBr3) first reacts with bromine (Br2) to form phosphorus bromide (PBr3) and bromide ions (Br–).The bromide ion then acts as a nucleophile attacking the carboxylic acid, displacing the hydroxyl group and forming an acyl bromide intermediate (RCOBr) and hydrogen bromide (HBr).
- Reaction of the Acyl Bromide Intermediate with Carboxylic Acid:
- The acyl bromide intermediate (RCOBr) reacts further with another molecule of carboxylic acid (RCOOH).In this step, the carboxylic acid acts as a nucleophile, attacking the carbonyl carbon of the acyl bromide.This nucleophilic attack displaces bromide ion, regenerating the carboxylic acid and forming the α-bromo carboxylic acid (RCOBr).
Where R and R’ represent alkyl or aryl groups.
The overall mechanism of the HVZ bromination reaction involves nucleophilic substitution at the carbonyl carbon of the carboxylic acid, leading to the formation of an α-bromo carboxylic acid. The use of phosphorus tribromide facilitates the generation of the acyl bromide intermediate, which is crucial for the selectivity and efficiency of the reaction.
The Hell-Volhard-Zelinsky (HVZ) bromination reaction has several important applications in organic synthesis due to its ability to selectively brominate α-carbon atoms of carboxylic acids. Some of the key applications include:
- Synthesis of α-Bromo Carboxylic Acids: The primary application of HVZ bromination is the preparation of α-bromo carboxylic acids. These compounds serve as versatile intermediates for further organic transformations. The α-bromo functionality can undergo various reactions such as nucleophilic substitution, elimination, and rearrangement, allowing for the synthesis of diverse organic compounds.
- Preparation of α-Hydroxy Carboxylic Acids: α-Bromo carboxylic acids obtained from HVZ bromination can be easily converted to α-hydroxy carboxylic acids through nucleophilic substitution with hydroxide ions (OH^-). This conversion is useful for synthesizing compounds such as lactic acid derivatives, which are important in pharmaceutical and industrial applications.
- Synthesis of α-Amino Acids: α-Bromo carboxylic acids can also serve as precursors for the synthesis of α-amino acids. By treating the α-bromo carboxylic acid with ammonia or an amine nucleophile, nucleophilic substitution leads to the formation of α-amino acids. This strategy is commonly employed in peptide synthesis and the preparation of pharmaceuticals.
- Functional Group Interconversions: The α-bromo functionality introduced by HVZ bromination can undergo various functional group interconversions, enabling the synthesis of diverse compounds. For example, α-bromo carboxylic acids can be converted to corresponding ketones or aldehydes via nucleophilic substitution followed by decarboxylation. Additionally, the α-bromo group can be substituted with other functional groups through further organic reactions.
- Preparation of Building Blocks for Natural Product Synthesis: α-Bromo carboxylic acids obtained from HVZ bromination are valuable building blocks for the synthesis of natural products. The versatility of α-bromo carboxylic acids allows for the introduction of complex functionalities found in natural products, facilitating the total synthesis of biologically active compounds.
Overall, the HVZ bromination reaction is a powerful tool in organic synthesis, providing access to α-bromo carboxylic acids and their derivatives, which find widespread applications in pharmaceuticals, agrochemicals, and materials science.










