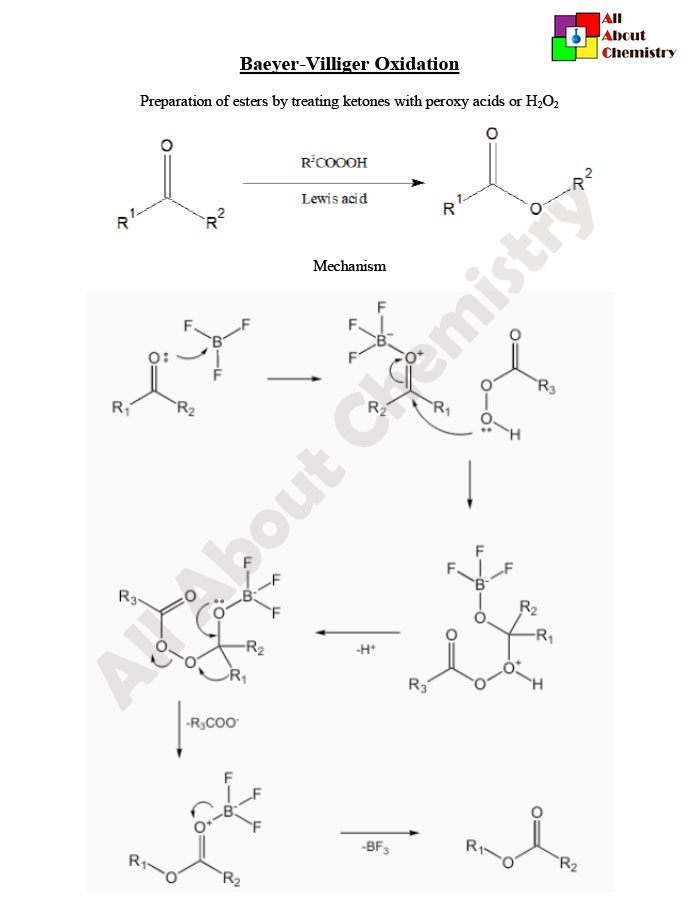
he Baeyer-Villiger oxidation is a chemical reaction that involves the oxidation of ketones or aldehydes to esters or lactones, respectively, using a peracid such as peracetic acid or meta-chloroperoxybenzoic acid (mCPBA). The reaction is named after the German chemists Adolf von Baeyer and Victor Villiger who independently discovered it in the late 19th and early 20th centuries.
The general reaction scheme for the Baeyer-Villiger oxidation is as follows:
R2C=O+R2′CO3H→R2C(=O)OR′+R2′CO2H
Where:
- R and R′ represent organic substituents
- R2C=O is a ketone or aldehyde
- R2C(=O)OR′ is an ester product
- R2′CO2H is a carboxylic acid byproduct
The Baeyer-Villiger oxidation mechanism involves several steps and can vary depending on the specific reactants and conditions. Here, I’ll outline a general mechanism for the Baeyer-Villiger oxidation using peracids like peracetic acid (CH3CO3H) as the oxidizing agent.
- Formation of the Peroxyacid: In the first step, the peracid is protonated to form a peroxycarboxylic acid. For instance, with peracetic acid, this step yields the peracetic acid (CH3CO3H).
CH3COOH+H2O2→CH3CO3H+H2O
- Formation of the Acyl Peroxide: The carbonyl compound (ketone or aldehyde) reacts with the peracid to form an acyl peroxide intermediate. This step involves the nucleophilic attack of the oxygen in the peracid on the carbonyl carbon of the ketone or aldehyde. This step is usually the rate-determining step of the reaction.
R2C=O+CH3CO3H→R2C(OOH)CH3+CH3CO2H
- Migration of the Alkyl Group: In the next step, rearrangement occurs where one of the alkyl groups migrates to the oxygen atom, breaking one of the carbon-oxygen bonds and forming a new carbon-oxygen bond. This rearrangement can result in the formation of either an ester or a lactone, depending on the nature of the starting carbonyl compound and the reaction conditions.
R2C(OOH)CH3→R2C(=O)CH2OOH
- Acid or Base Catalyzed Hydrolysis: The final step involves hydrolysis of the intermediate ester or lactone under acidic or basic conditions to yield the final product – an ester or a lactone, respectively, along with a carboxylic acid.
R2C(=O)CH2OOH+H2O→R2C(=O)OR+HO2CH2CO2H
This mechanism showcases how a ketone or aldehyde is transformed into an ester or a lactone via the Baeyer-Villiger oxidation. It’s worth noting that the exact mechanism might vary slightly depending on the specific reactants and reaction conditions. Additionally, other peracids such as meta-chloroperoxybenzoic acid (mCPBA) can also be used as oxidizing agents in the Baeyer-Villiger oxidation, and the mechanism might differ slightly when using different peracids.
The Baeyer-Villiger oxidation is a versatile synthetic tool with numerous applications in organic chemistry. Some of its key applications include:
- Functional Group Transformations: The Baeyer-Villiger oxidation allows for the conversion of ketones and aldehydes into esters and lactones, respectively. This transformation is valuable for introducing oxygen-containing functional groups into organic molecules, thereby increasing molecular complexity and providing access to a wide range of structurally diverse compounds.
- Synthesis of Lactones and Macrolides: The Baeyer-Villiger oxidation is commonly employed in the synthesis of lactones and macrolides, which are important classes of natural products and pharmaceutical compounds. By selectively oxidizing ketones or aldehydes adjacent to a carbonyl group within a molecule, the Baeyer-Villiger oxidation facilitates the construction of lactone rings, which are prevalent in many bioactive molecules.
- Stereochemical Control: The Baeyer-Villiger oxidation can be employed as a stereochemically selective transformation, allowing for the introduction of chirality into organic molecules. Depending on the specific substrate and reaction conditions, the migration of alkyl groups during the oxidation process can occur with high stereocontrol, enabling the synthesis of enantiomerically enriched compounds.
- Synthesis of Fragrances and Flavors: The Baeyer-Villiger oxidation is widely utilized in the synthesis of fragrance and flavor compounds. By incorporating lactones into the molecular structure of aromatic compounds, this oxidation reaction contributes to the creation of characteristic odor and taste profiles in perfumes, cosmetics, and food products.
- Natural Product Synthesis: The Baeyer-Villiger oxidation plays a crucial role in the synthesis of various natural products, including antibiotics, antifungals, and anticancer agents. By enabling the construction of complex molecular frameworks containing oxygenated functional groups, this oxidation reaction facilitates the efficient synthesis of natural product analogs and derivatives for biological evaluation and drug discovery efforts.
- Polymer Chemistry: In polymer chemistry, the Baeyer-Villiger oxidation can be utilized to modify polymer backbones or side chains, leading to the introduction of functional groups that impart desired properties to the resulting polymers. This strategy enables the synthesis of tailored polymer materials with enhanced solubility, reactivity, or compatibility for specific applications in materials science and engineering.
Overall, the Baeyer-Villiger oxidation offers a powerful and versatile synthetic method for the construction of diverse organic molecules with applications spanning from pharmaceuticals and agrochemicals to materials science and fragrance chemistry. Its ability to selectively introduce oxygen functionality and control stereochemistry makes it a valuable tool for synthetic chemists seeking to access complex molecular architectures efficiently.








