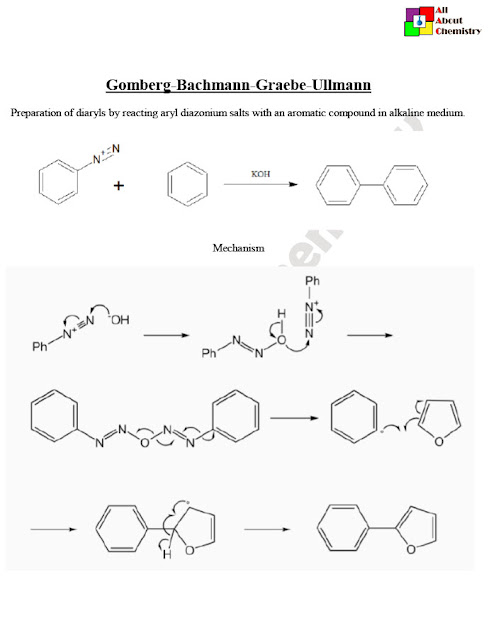
The Gomberg-Bachmann-Graebe-Ullmann (GBGU) reaction is a multistep organic synthesis used to prepare diaryl compounds, specifically biaryl compounds, from aryl halides (e.g., aryl bromides or aryl iodides) and aryl Grignard reagents in the presence of a copper(I) catalyst. This reaction is named after the chemists who contributed to its development: Moses Gomberg, Wilhelm Bachmann, Carl Graebe, and Friedrich Ullmann.
The general reaction scheme for the Gomberg-Bachmann-Graebe-Ullmann reaction is as follows:
ArX+ArMgX + Cu(I) catalyst → Ar-Ar+MgXCl
Where ArX is an aryl halide (e.g., aryl bromide or aryl iodide), ArMgX is an aryl Grignard reagent, Ar-Ar represents the desired biaryl product, and MgXCl is a magnesium halide byproduct.
The mechanism of the Gomberg-Bachmann-Graebe-Ullmann (GBGU) reaction involves several steps, including transmetalation, oxidative addition, transmetalation, and reductive elimination. Here’s a detailed mechanism of the GBGU reaction:
- Formation of Aryl Copper(I) Intermediate:
- Initially, the aryl Grignard reagent (ArMgX) reacts with the copper(I) catalyst to form an aryl copper(I) complex. ArMgX+Cu(I)→ArCu+MgXCl
- Oxidative Addition:
- The aryl halide (ArX) undergoes oxidative addition with the aryl copper(I) complex, leading to the formation of an aryl copper(III) intermediate. ArCu+ArX→ArCu(III)X
- Transmetalation:
- Another molecule of the aryl Grignard reagent (ArMgX) reacts with the aryl copper(III) intermediate, leading to the transfer of the aryl group to the copper center. ArMgX+ArCu(III)X→ArArCu+MgXCl
- Reductive Elimination:
- The aryl-aromatic copper complex undergoes reductive elimination to yield the desired biaryl product and regenerate the copper(I) catalyst. ArArCu→Ar-Ar+Cu(I)
Overall, the Gomberg-Bachmann-Graebe-Ullmann reaction proceeds through a sequence of steps involving the formation of aryl copper intermediates, oxidative addition of aryl halides, transmetalation reactions, and reductive elimination. This mechanism enables the construction of biaryl compounds from aryl halides and aryl Grignard reagents under copper catalysis.
The Gomberg-Bachmann-Graebe-Ullmann (GBGU) reaction has several important applications in organic synthesis and materials science due to its ability to form carbon-carbon bonds between aryl groups. Here are some of its key applications:
- Biaryl Synthesis: The GBGU reaction is widely used for the synthesis of biaryl compounds, which are important structural motifs in organic chemistry. Biaryls are found in a wide range of natural products, pharmaceuticals, agrochemicals, and materials. The GBGU reaction provides a versatile and efficient method for connecting two aryl groups together.
- Pharmaceutical Synthesis: Biaryl compounds are common structural elements in many pharmaceuticals due to their diverse biological activities. The GBGU reaction enables the synthesis of complex biaryl-based drug candidates and intermediates. It has been utilized in the synthesis of various pharmaceuticals, including antiviral agents, anticancer drugs, and antibiotics.
- Materials Science: Biaryl compounds are also valuable building blocks in materials science. They are used in the synthesis of organic electronic materials, such as OLEDs (organic light-emitting diodes), organic semiconductors, and conducting polymers. The GBGU reaction provides access to functionalized biaryl scaffolds that can be further modified for specific electronic and optical properties.
- Natural Product Synthesis: The GBGU reaction is employed in the synthesis of natural products and their analogs. Many natural products contain biaryl substructures, and the GBGU reaction offers a powerful strategy for their construction. By accessing complex biaryl motifs via the GBGU reaction, chemists can streamline the synthesis of natural products and explore structure-activity relationships.
- Catalysis and Methodology Development: The GBGU reaction has served as a model system for the development of new catalytic methodologies in organic synthesis. Researchers continue to explore novel catalyst systems, reaction conditions, and substrate scopes to expand the utility and efficiency of the GBGU reaction and related cross-coupling reactions.
Overall, the Gomberg-Bachmann-Graebe-Ullmann reaction is a versatile tool in organic synthesis with broad applications in pharmaceuticals, materials science, natural product synthesis, and catalysis. Its ability to form biaryl compounds has significantly impacted various fields of chemistry and continues to inspire innovative research and applications.








