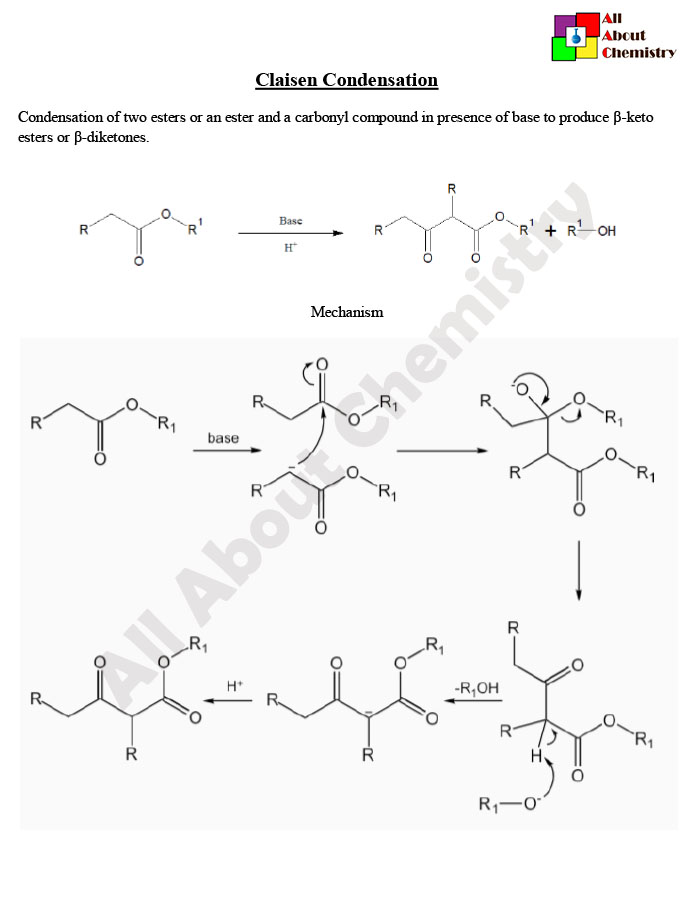
The Claisen condensation is a classic organic reaction named after the German chemist Rainer Ludwig Claisen. It involves the formation of a β-ketoester or β-diketone by the condensation of two ester or ketone molecules in the presence of a strong base. The reaction proceeds via an aldol-type condensation mechanism and is an important tool in organic synthesis for the formation of carbon-carbon bonds.
The general reaction scheme for the Claisen condensation is as follows:
R1-CO-CH2-R2+R3-CO-CH2-R4+Base→R1-CO-CH2-CH2-CO-CH2-R4+ROH
In this reaction, two ester (or ketone) molecules, often referred to as the “Claisen partners,” react in the presence of a strong base to form a β-ketoester or β-diketone. The base typically used is an alkoxide ion (RO−), generated by the deprotonation of an alcohol (often an alcohol solvent such as ethanol or methanol).
The mechanism of the Claisen condensation involves several steps:
- Deprotonation: The base (alkoxide ion, RO−) abstracts a proton from the carbonyl carbon of one ester molecule, forming an enolate ion.
- Nucleophilic Attack: The enolate ion acts as a nucleophile and attacks the carbonyl carbon of another ester molecule, leading to the formation of a tetrahedral intermediate.
- Proton Transfer and Elimination: A proton transfer occurs, leading to the formation of the β-ketoester or β-diketone product. Simultaneously, the alkoxide ion is regenerated, completing the catalytic cycle.
The Claisen condensation is widely used in organic synthesis to construct complex molecules with extended carbon frameworks. It is particularly valuable for the synthesis of β-ketoesters and β-diketones, which are versatile intermediates for the synthesis of pharmaceuticals, natural products, and other fine chemicals. Additionally, variants of the Claisen condensation, such as the Dieckmann condensation and the Crossed Claisen condensation, offer additional synthetic strategies for achieving diverse chemical transformations.
The Claisen condensation is a versatile synthetic tool in organic chemistry, offering numerous applications in the preparation of various compounds. Here are some of its key applications:
- β-Ketoesters and β-Diketones Synthesis: One of the primary applications of the Claisen condensation is in the synthesis of β-ketoesters and β-diketones. These compounds serve as important intermediates in organic synthesis for the preparation of various functionalized molecules.
- Synthesis of Pharmaceuticals: β-Ketoesters and β-diketones synthesized via the Claisen condensation are often key intermediates in the synthesis of pharmaceutical compounds. These compounds can undergo further functionalization to introduce specific pharmacophores, enabling the synthesis of diverse drug candidates.
- Natural Product Synthesis: The Claisen condensation is frequently used in the synthesis of natural products, many of which contain β-ketoester or β-diketone functionalities. By employing the Claisen condensation, chemists can access complex natural product scaffolds and functional groups, facilitating the total synthesis of natural products with diverse biological activities.
- Polyketide Synthesis: Polyketides are a class of natural products with diverse biological activities, including antibiotics, antifungals, and anticancer agents. The Claisen condensation plays a crucial role in the biosynthesis of polyketides and is also used in the laboratory synthesis of polyketide analogs for drug discovery and development.
- Construction of Carbon-Carbon Bonds: The Claisen condensation enables the formation of carbon-carbon bonds, allowing chemists to construct complex molecular architectures. It is particularly useful for the synthesis of molecules with extended conjugation or multiple functional groups.
- Regioselective Synthesis: The Claisen condensation can exhibit regioselectivity, leading to the preferential formation of one product over another. This regioselectivity can be controlled by the choice of reactants, reaction conditions, and the presence of directing groups, enabling the synthesis of specific regioisomers.
- Asymmetric Synthesis: Asymmetric variants of the Claisen condensation have been developed, allowing for the synthesis of enantioenriched β-ketoesters and β-diketones. Chiral auxiliaries or catalysts are employed to control the stereochemistry of the reaction, enabling the synthesis of enantiomerically pure compounds.
- Functional Group Interconversion: The Claisen condensation can be used to interconvert functional groups in organic molecules. By selecting appropriate reactants and reaction conditions, chemists can introduce or modify functional groups, facilitating the synthesis of diverse organic compounds.
Overall, the Claisen condensation is a versatile synthetic method with broad applications in organic synthesis, pharmaceutical chemistry, natural product synthesis, and materials science. Its ability to form carbon-carbon bonds and access complex molecular architectures makes it an indispensable tool for synthetic chemists.








