The Amadori Glucoamine rearrangement is a chemical reaction involving the rearrangement of aminosugars, particularly glucose and its derivatives. This reaction is named after the Italian chemist Mario Amadori, who first described it in 1925.
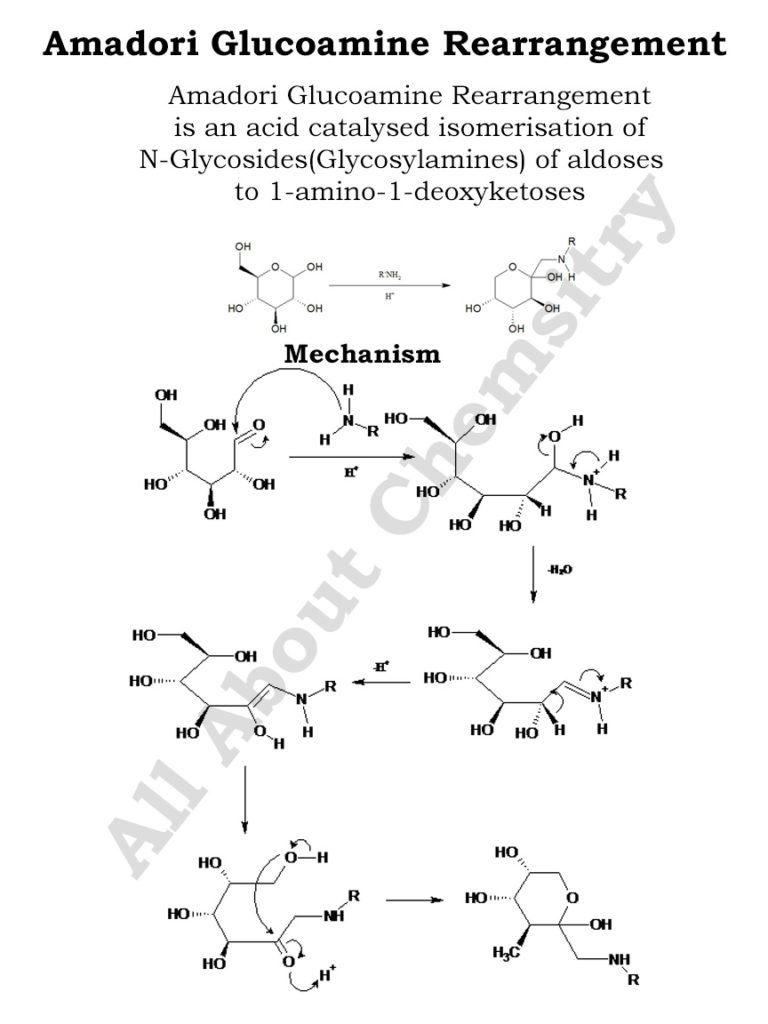
The reaction involves the initial condensation of a reducing sugar with an amino compound, typically an amino acid or a protein, to form a Schiff base. This Schiff base undergoes a series of rearrangements, resulting in the formation of a ketoamine or an Amadori product.
The Amadori product is a key intermediate in the Maillard reaction, which is a complex cascade of chemical reactions that occur between reducing sugars and amino compounds during heating or cooking. This reaction pathway leads to the formation of numerous flavor and aroma compounds, as well as brown pigments, in foods.
The Amadori rearrangement plays a significant role in food chemistry, particularly in processes like caramelization and flavor development during cooking and food processing. Additionally, it has implications in the field of biochemistry, particularly in the context of glycation reactions and the formation of advanced glycation end-products (AGEs), which are associated with various pathologies including diabetes and aging-related diseases.
The Amadori rearrangement mechanism involves several steps and is a complex process. It’s important to note that while the general mechanism is understood, the exact details may vary depending on the specific reaction conditions and the nature of the reactants involved. Here’s a simplified overview of the mechanism:
- Formation of Schiff base: The reaction begins with the condensation of a reducing sugar (such as glucose) with an amino compound (such as an amino acid or a protein), leading to the formation of a Schiff base. This step involves the nucleophilic attack of the amino group on the carbonyl carbon of the sugar, followed by dehydration to form the Schiff base.
- Proton Shift and Rearrangement: In the presence of water or other solvent molecules, the Schiff base undergoes a proton shift, leading to the migration of the amino group to the adjacent carbon atom. This step results in the formation of an N-substituted intermediate.
- Tautomerization and Rearrangement: The N-substituted intermediate undergoes tautomerization, resulting in the migration of a hydrogen atom to the adjacent carbon atom along with rearrangement of the carbon-carbon double bond. This step leads to the formation of an Amadori product, typically a ketoamine.
- Further Rearrangements and Products: The Amadori product can undergo further rearrangements and reactions, depending on the specific conditions and the nature of the reactants involved. In the context of the Maillard reaction, the Amadori product serves as a precursor for the formation of various flavor compounds, aroma compounds, and brown pigments through further chemical reactions.
The Amadori rearrangement, particularly in the context of glucose and amino compounds, has several important applications in various fields:
- Food Chemistry: The Amadori rearrangement is a key step in the Maillard reaction, which is responsible for the browning of foods during cooking and processing. This reaction pathway leads to the formation of a wide range of flavor compounds, aroma compounds, and brown pigments, contributing to the sensory properties of cooked foods. Understanding and controlling the Maillard reaction is essential for food scientists and chefs to develop desired flavors and colors in foods.
- Food Processing: Knowledge of the Amadori rearrangement is essential in food processing industries to optimize the production of desirable flavor and color compounds in processed foods. Control of reaction conditions such as temperature, pH, and moisture content can influence the extent and nature of the Maillard reaction, thereby affecting the final product quality.
- Flavor Enhancement: The understanding of the Amadori rearrangement and the Maillard reaction is utilized in the food industry to develop flavor enhancers and food additives. These compounds can be used to impart specific flavors or enhance existing flavors in food products.
- Biomedical Research: In the field of biomedical research, the Amadori rearrangement has implications in the study of glycation reactions and the formation of advanced glycation end-products (AGEs). AGEs are associated with various pathologies, including diabetes, aging-related diseases, and neurodegenerative disorders. Understanding the mechanisms of Amadori rearrangement and AGE formation can provide insights into disease mechanisms and potential therapeutic interventions.
- Chemical Synthesis: The principles underlying the Amadori rearrangement can be applied in organic synthesis for the preparation of various amino-sugar derivatives and related compounds. These compounds find applications in pharmaceuticals, agrochemicals, and materials science.
Overall, the Amadori rearrangement has broad applications in food chemistry, food processing, flavor enhancement, biomedical research, and chemical synthesis, making it a topic of significant interest and importance in various fields.








