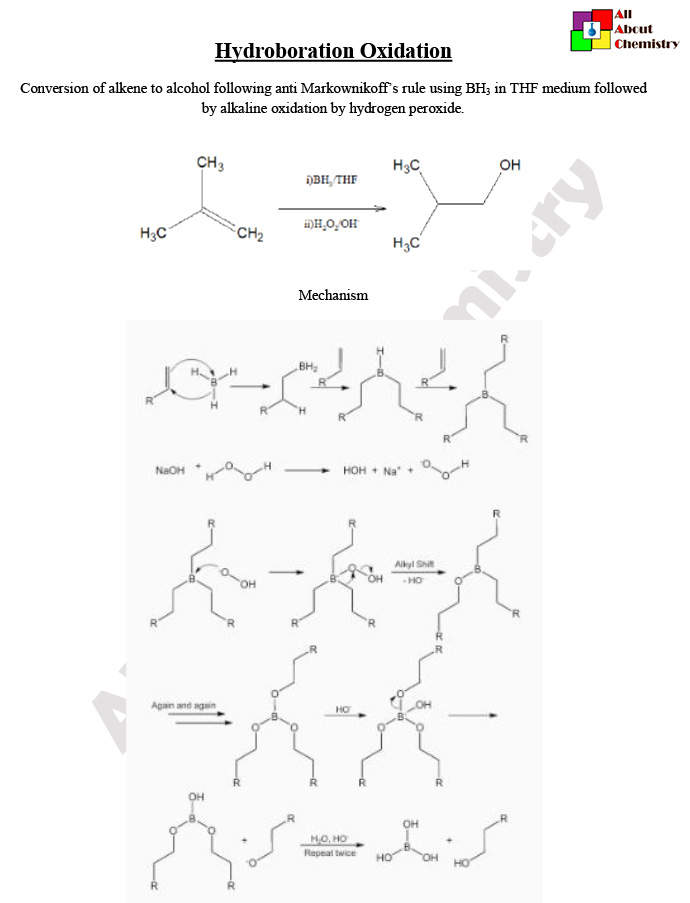
Hydroboration-oxidation is a chemical reaction used to convert alkenes (unsaturated hydrocarbons with carbon-carbon double bonds) into alcohols.
The overall reaction can be summarized as follows:
Alkene+Borane+Oxidizing Agent→Alcohol
The mechanism of the hydroboration-oxidation reaction involves two main steps: hydroboration and oxidation. Here’s a general overview of the mechanism:
- Hydroboration:
- The hydroboration reaction begins with the alkene (RCH=CH2) and borane (BH3) in the presence of a solvent such as tetrahydrofuran (THF).
- Initially, the alkene undergoes coordination with the borane molecule. This results in the formation of a cyclic transition state in which the boron atom forms a bond with one of the carbon atoms of the double bond, while the other carbon atom retains its pi bond to hydrogen. This step is often referred to as syn addition because the boron and hydrogen atoms add to the same face of the double bond.
- As a result of this coordination, a four-membered cyclic intermediate known as a borane complex or boronate ester is formed. The boron atom in this complex has a vacant p-orbital and a partial positive charge.
- Next, the alkene coordinates to another molecule of borane, resulting in the formation of a diborane intermediate (RCH2-BH2). This intermediate is relatively unstable and undergoes rearrangement to a more stable trialkylborane intermediate (R3B).
- The final step of hydroboration involves the protonation of the trialkylborane intermediate by a proton source (such as water or alcohol). This leads to the formation of the desired boron-containing alcohol intermediate.
- Oxidation:
- In the oxidation step, the boron-containing alcohol intermediate undergoes reaction with an oxidizing agent such as hydrogen peroxide (H2O2) or alkaline potassium permanganate (KMnO4).
- The oxidation process involves the replacement of the boron atom in the boron-containing alcohol intermediate with a hydroxyl group (OH). This results in the formation of the final alcohol product.
- The mechanism of the oxidation step can vary depending on the choice of oxidizing agent. For example, hydrogen peroxide typically oxidizes the boron-containing alcohol through a two-electron transfer process, while alkaline potassium permanganate undergoes a more complex oxidation-reduction reaction.
Overall, the hydroboration-oxidation mechanism proceeds through a series of coordinated reactions involving the alkene, borane, solvent, and oxidizing agent to yield the desired alcohol product. The reaction is highly regioselective and stereoselective, and it provides excellent yields of anti-Markovnikov alcohols with syn addition of the boron and hydrogen atoms across the carbon-carbon double bond.
Hydroboration-oxidation is a versatile and widely used method in organic synthesis for the conversion of alkenes (unsaturated hydrocarbons with carbon-carbon double bonds) into alcohols. This reaction has several important applications in organic chemistry:
- Anti-Markovnikov Alcohol Synthesis: One of the significant advantages of hydroboration-oxidation is its anti-Markovnikov selectivity. The boron atom adds to the less substituted carbon atom of the double bond, leading to the formation of anti-Markovnikov alcohols. This selectivity is valuable for the synthesis of alcohols with specific regiochemical control, particularly in cases where traditional Markovnikov-selective reactions would not yield the desired product.
- Functional Group Compatibility: Hydroboration-oxidation is compatible with a wide range of functional groups, including halides, carbonyls, nitriles, and ethers. This compatibility allows for the synthesis of complex molecules containing multiple functional groups without undesired side reactions or complications.
- Stereochemical Control: The hydroboration-oxidation reaction proceeds with syn addition of the boron and hydrogen atoms across the carbon-carbon double bond. This syn stereochemistry provides excellent control over the stereochemistry of the resulting alcohol product, making it useful for the synthesis of stereochemically pure compounds and natural products.
- Mild Reaction Conditions: Hydroboration-oxidation typically proceeds under mild reaction conditions, making it suitable for a wide range of substrates and functional groups. The reaction can be carried out at room temperature or slightly elevated temperatures and does not require harsh reaction conditions or specialized equipment.
- Synthetic Versatility: Hydroboration-oxidation can be used for the synthesis of various types of alcohols, including primary, secondary, and tertiary alcohols, as well as cyclic alcohols and heteroatom-containing alcohols. Additionally, it can be applied to the synthesis of complex molecules, such as pharmaceuticals, natural products, and fine chemicals.
Overall, hydroboration-oxidation is a valuable tool in organic synthesis, offering excellent regioselectivity, compatibility with diverse functional groups, stereochemical control, and mild reaction conditions. Its versatility and reliability make it a preferred method for the synthesis of alcohols and the construction of complex organic molecules in both academic and industrial settings.










