Aza-Achmatowicz Reaction or Aza-Achmatowicz Rearrangement is the oxidative rearrangement of furanylamines or furylacetamides to hydropyridone.
The Aza-Achmatowicz rearrangement is a chemical reaction named after its discoverers, Polish chemists Bogdan Achmatowicz Jr. and Bogdan Achmatowicz Sr. It is a valuable synthetic transformation used in organic chemistry to construct nitrogen-containing heterocycles from readily available starting materials.
The reaction involves the conversion of an alpha-hydroxyketone or alpha-hydroxyaldehyde to an alpha-amino ketone or alpha-aminoaldehyde, respectively, through a series of intramolecular transformations. The rearrangement typically proceeds under acidic conditions, and the resulting products often contain a bicyclic or spirocyclic amine motif.
The Aza-Achmatowicz rearrangement proceeds through several steps involving acid-catalyzed cyclization, ring expansion, tautomerization, and rearrangement. Here’s a proposed mechanism for the Aza-Achmatowicz rearrangement:
- Acid-Catalyzed Cyclization: The reaction begins with protonation of the carbonyl oxygen atom of the alpha-hydroxyketone or alpha-hydroxyaldehyde, activating the carbonyl group towards nucleophilic attack. This protonation step is facilitated by an acid catalyst, typically a strong mineral acid such as sulfuric acid (H2SO4).
R1−C(OH)−CH2−C(O)R2+H+→R1−C(OH)−CH2+−C(O)R2+H2O
- Ring Expansion: The activated carbonyl group undergoes intramolecular nucleophilic attack by the adjacent hydroxyl group, leading to the formation of an oxonium intermediate. Subsequently, a ring expansion occurs, with migration of the carbon-oxygen bond adjacent to the hydroxyl group to form a new carbon-nitrogen bond.
R1−C(OH)−CH2+−C(O)R2→R1−C(OH)−CH(OH)−C(O)R2
R1−C(OH)−CH(OH)−C(O)R2→R1−C(OH)−CH(NH2)−C(O)R2
- Tautomerization: The resulting lactam intermediate undergoes tautomerization, involving migration of a proton from the nitrogen atom to the adjacent carbon atom, accompanied by rearrangement of electrons.
R1−C(OH)−CH(NH2)−C(O)R2→R1−C(NH2)−CH(OH)−C(O)R2
- Rearrangement: Finally, the tautomeric form undergoes a rearrangement, leading to the migration of the amino group and formation of the desired product containing a nitrogen-containing heterocyclic motif.
R1−C(NH2)−CH(OH)−C(O)R2→R1−C(NH2)−CH(NH2)−C(O)R2
Overall, the Aza-Achmatowicz rearrangement involves acid-catalyzed cyclization, ring expansion, tautomerization, and rearrangement steps, ultimately leading to the formation of nitrogen-containing heterocycles from alpha-hydroxyketones or alpha-hydroxyaldehydes.
Mechanism:

Application:

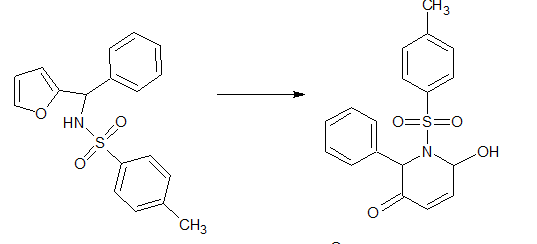
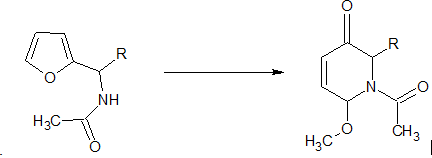
The Aza-Achmatowicz rearrangement is a versatile synthetic tool in organic chemistry, offering a straightforward method for the construction of nitrogen-containing heterocycles from readily available starting materials. Here are some of its key applications:
- Total Synthesis of Natural Products: The Aza-Achmatowicz rearrangement has been widely utilized in the total synthesis of various natural products containing nitrogen heterocycles. These natural products often exhibit diverse biological activities and have important pharmacological properties. By incorporating the Aza-Achmatowicz rearrangement as a key step in synthetic routes, chemists can efficiently access complex molecular architectures.
- Medicinal Chemistry: Nitrogen-containing heterocycles are prevalent motifs in many biologically active compounds and pharmaceuticals. The Aza-Achmatowicz rearrangement provides a practical method for the synthesis of such heterocycles, making it valuable in medicinal chemistry for the preparation of drug candidates and their analogs. Researchers have employed this rearrangement to synthesize libraries of compounds for drug discovery and optimization studies.
- Methodology Development: The study of the Aza-Achmatowicz rearrangement has led to the development of new methodologies in organic synthesis. Chemists have explored variations of the reaction conditions, substrate scope, and catalyst systems to expand the utility and efficiency of the rearrangement. These advancements contribute to the development of novel synthetic strategies and enable the synthesis of complex heterocyclic scaffolds.
- Diversity-Oriented Synthesis (DOS): The Aza-Achmatowicz rearrangement can be integrated into diversity-oriented synthesis (DOS) strategies to generate structurally diverse compound libraries. DOS aims to synthesize large collections of diverse compounds for biological screening and lead discovery. By incorporating the Aza-Achmatowicz rearrangement into DOS protocols, chemists can rapidly access diverse nitrogen-containing heterocycles for evaluation in various biological assays.
- Materials Science: Nitrogen-containing heterocycles synthesized via the Aza-Achmatowicz rearrangement have applications beyond medicinal chemistry. They are used as building blocks in materials science for the synthesis of functional materials, such as polymers, dendrimers, and organic semiconductors. The ability to access diverse heterocyclic motifs enables the design and synthesis of materials with tailored properties for applications in electronics, optics, and catalysis.
Overall, the Aza-Achmatowicz rearrangement is a valuable synthetic tool with broad applications in organic synthesis, medicinal chemistry, methodology development, diversity-oriented synthesis, and materials science. Its versatility and efficiency make it an indispensable method for the synthesis of nitrogen-containing heterocycles with diverse applications in science and technology.








