Organic conversions refer to chemical reactions or processes in which one organic compound is transformed into another through the manipulation of its chemical structure. There are numerous types of organic conversions, each serving specific purposes in organic synthesis, drug discovery, materials science, and other fields. Here are some common types of organic conversions along with examples:
- Functional Group Interconversions: These conversions involve changing one functional group into another. For instance:
- Conversion of alcohols to alkyl halides through substitution reactions.
- Oxidation of alcohols to aldehydes or ketones.
- Reduction of carbonyl compounds to alcohols.
- Halogenation of alkenes to form alkyl halides.
- Protection and Deprotection Reactions: In multi-step synthesis, protecting groups are often used to temporarily block certain functional groups, preventing them from reacting during subsequent steps. Examples include:
- Acetal formation to protect carbonyl groups.
- Silyl ether formation to protect alcohols.
- Deprotection of acetals or silyl ethers using acidic conditions.
- Carbon-Carbon Bond Formation: These conversions involve the formation of new carbon-carbon bonds. Examples include:
- Aldol condensation to form β-hydroxy carbonyl compounds.
- Grignard reaction to form carbon-carbon bonds between an organomagnesium halide and a carbonyl compound.
- Heck reaction for the formation of carbon-carbon bonds between aryl or vinyl halides and alkenes.
- Rearrangement Reactions: These involve the rearrangement of atoms within a molecule to form a different structural isomer. Examples include:
- Wagner-Meerwein rearrangement involving migration of a hydride, alkyl, or aryl group.
- Beckmann rearrangement converting oximes to amides.
- Claisen rearrangement leading to the rearrangement of allyl vinyl ethers to form allyl ketones.
- Ring-Opening and Ring-Closing Reactions: These conversions involve breaking or forming cyclic structures. Examples include:
- Ring-opening of epoxides with nucleophiles to form diols.
- Ring-closing metathesis (RCM) for the synthesis of cyclic compounds using olefin metathesis.
- Diels-Alder reaction for the formation of cyclic compounds from dienes and dienophiles.
These are just a few examples of the many organic conversions used in organic chemistry. Each type of conversion offers a unique way to modify or synthesize organic molecules for various applications.
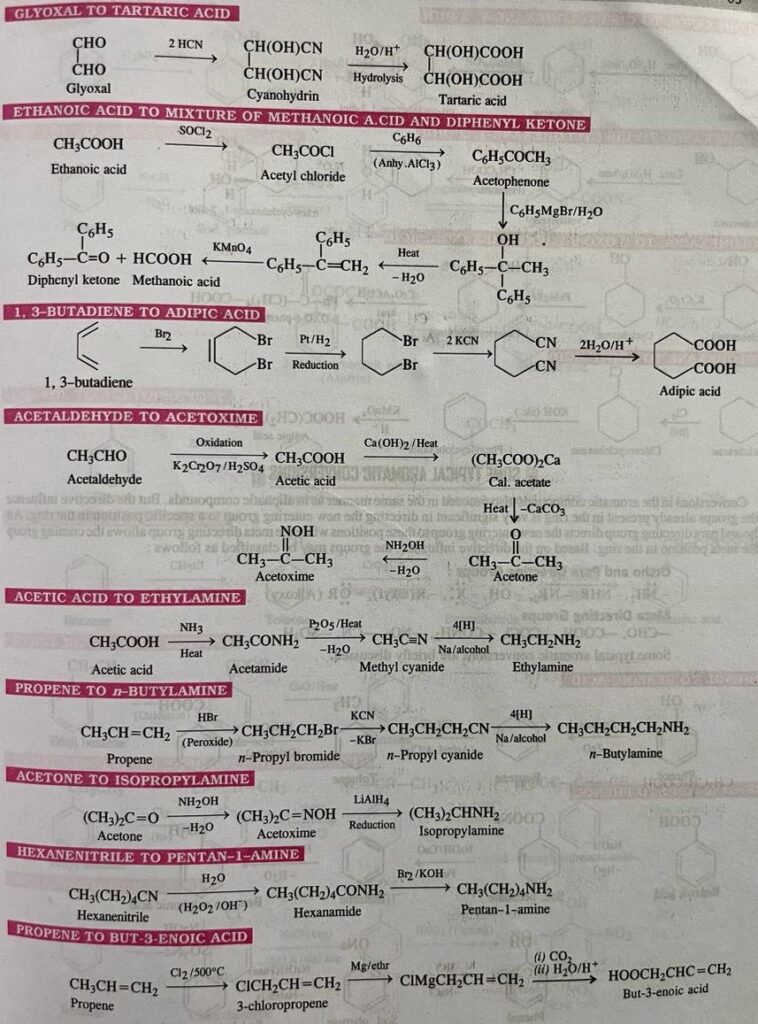
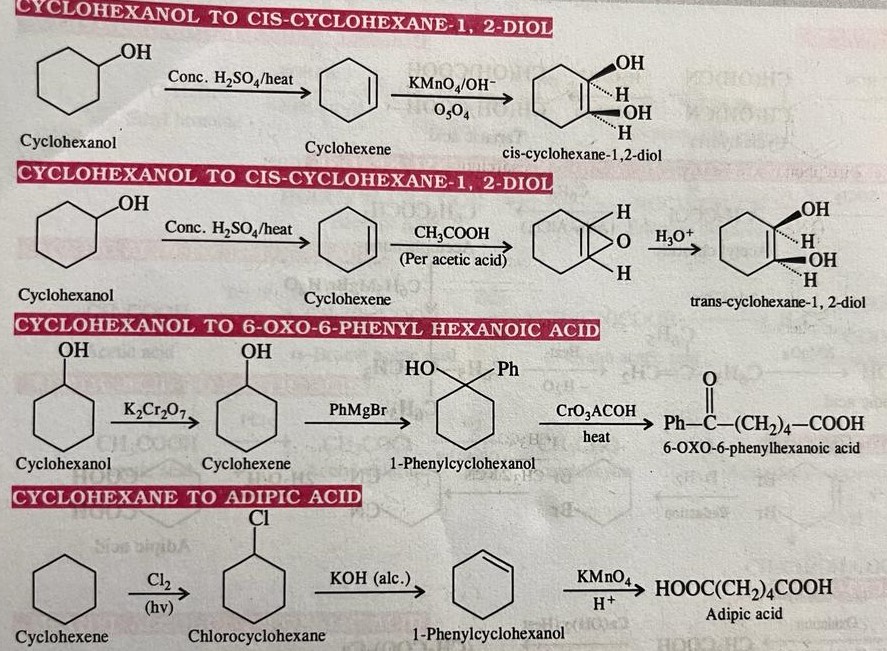
We have tried to provide some common organic conversions –
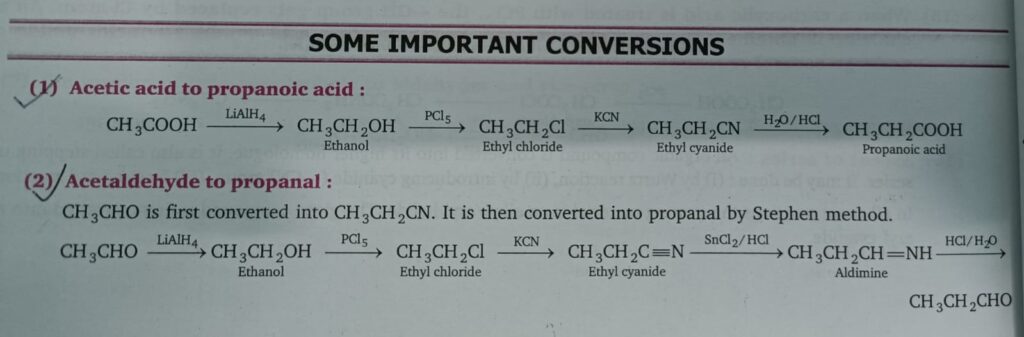
- Acetic acid to propanoic acid
- Acetaldehyde to propanal
- Ethyl bromide to n-propyl bromide
- methyl iodide to ethyl iodide
- .
- .
- Methanol to acetone
- Ethanol to propanone
- Propanone to propene
- Ethanol to ethane diol
- ethanol to 1,1-dichloro ethane
- Alkyl halide to ether
- Methyl bromide to ethyl amine
- 1-propanol to 2-propanol
- 2-propanol to 1-propanol
- Isopropyl alcohol to tert alcohol
- .
- ethanol to 1-propanol
- Propanoic acid to acetic acid
- Formic acid to acetic acid
- Propanal to ethane
- Propene to 1-propyl alcohol
- Acetic acid to butane
- Acetaldehyde to methyl bromide
- Acetaldehyde to crotonaldehyde
- Acetaldehyde to acetone
- Acetaldehyde to formaldehyde
- Acetaldehyde to ethyl amine
- Ethyne to acetic acid
- Methane to ethanol
- Acetic acid to acetaldehyde
- Methane to acetone
- Acetic acid to ethyl amine
- Acetic acid to methyl alcohol
- .
- Isocyanide to secondary amine
- Acetaldehyde to butane-1,3-diol
- Ethanol to 3-hydroxy butanal
- Methyl bromide to acetic acid
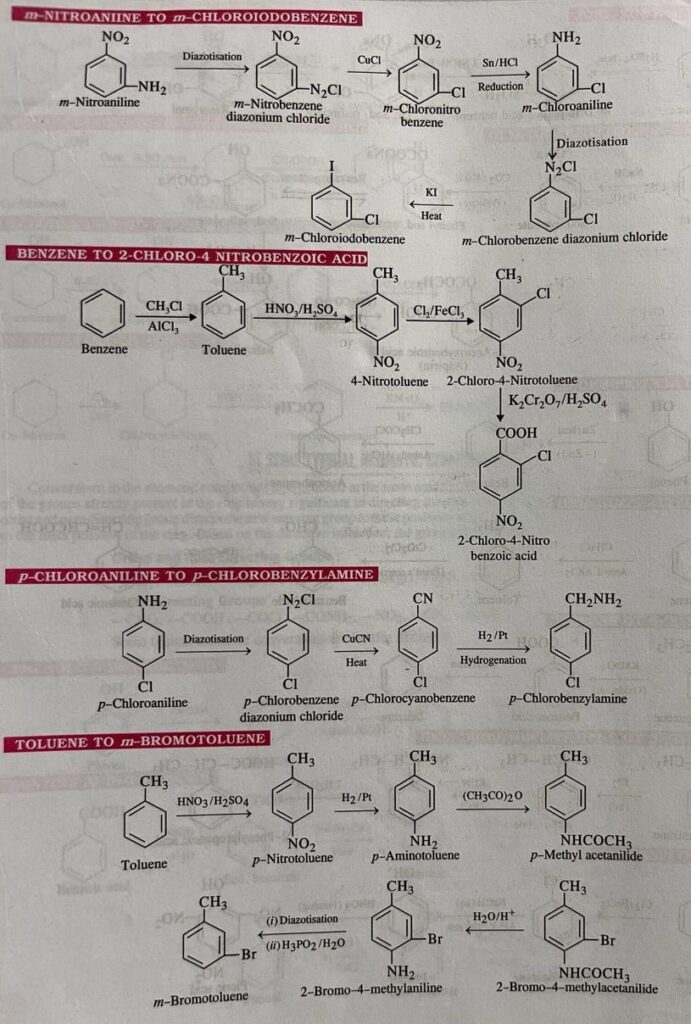
- Nitrobenzene to benzoic acid
- Aniline to benzylalcohol
- Nitrobenzene to iodobenzene
- Aniline to chlorobenzene
- Aniline fluorobenzene
- Aniline to benzene
- Aniline to phenyl cyanide
- Aniline to benzoic acid
- Nitrobenzene to benzene
- Nitrobenzene to 2,4,6-tribromo aniline
- Benzaldehyde to acetophenone
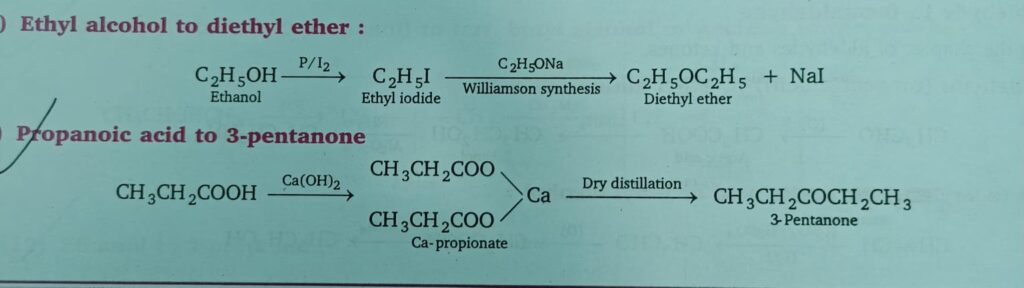
- Ethyl alcohol to diethyl ether
- Propanoic acid to 3-pentanone
- Benzaldehyde to benzophenone
- Benzaldehyde to cyanobenzene
- Benzaldehyde to cinnammic acid
- Benzaldehyde to nitrobenzene
- Benzoic acid to aniline
- Benzoic acid to benzaldehyde
- Benzoic acid to phenyl cyanide
- Benzoic acid to benzamide
- Salicylic acid to benzene
- Benzene to diphenyl
- Benzoic acid to benzyl chloride
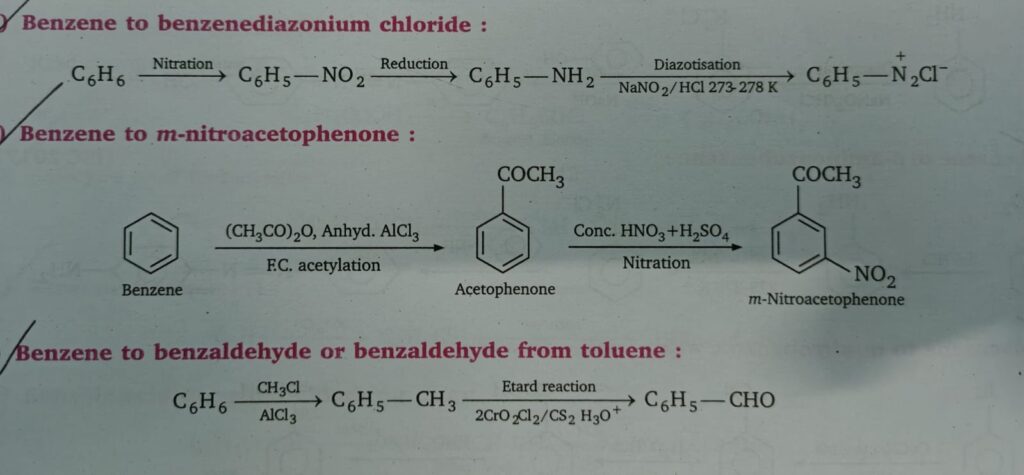
- Benzene to diazonium chloride
- Benzene to m-nitroacetophenone
- Benzene to benzaldehyde
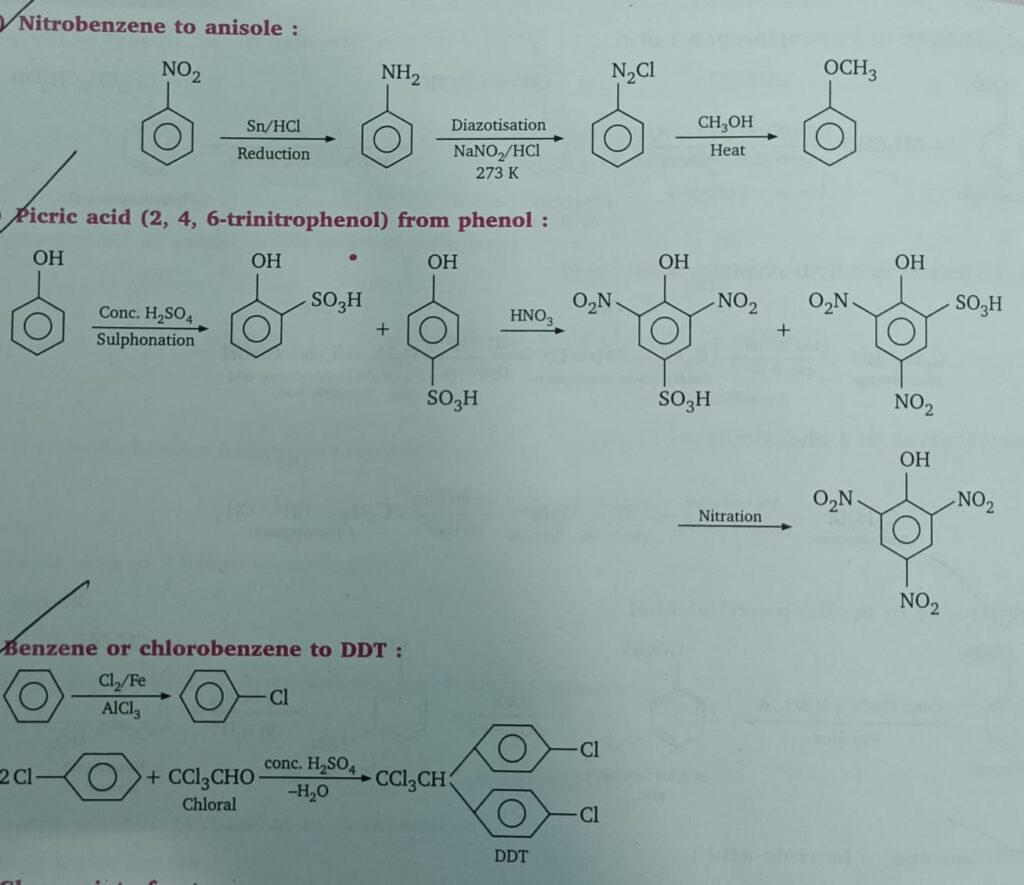
- Nitrobenzene to anisole
- Phenol to picric acid
- Benzene to DDT
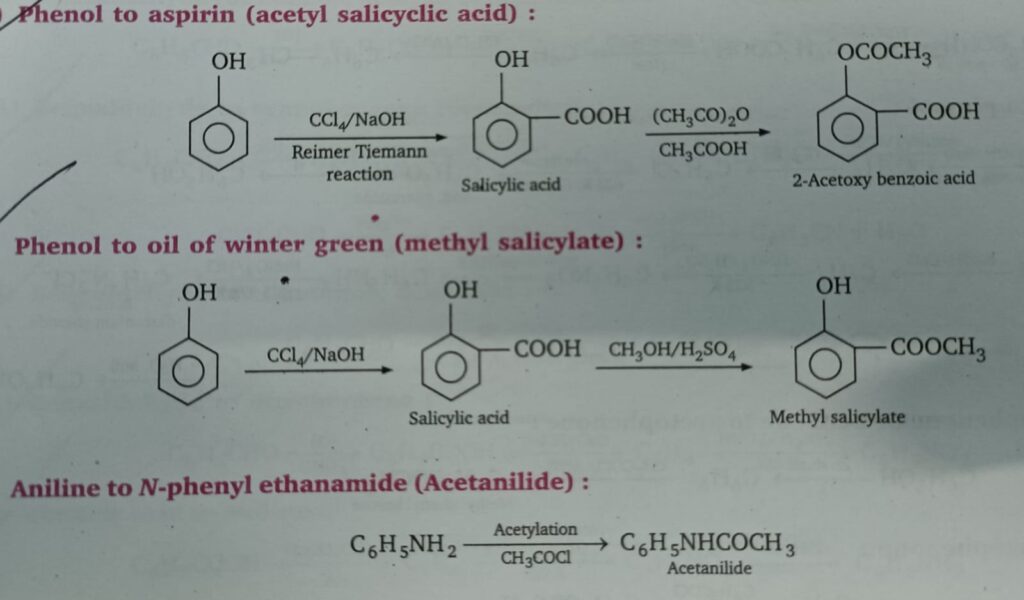
- Phenol to aspirin
- Phenol to methyl salicylate
- Aniline to acetanilide
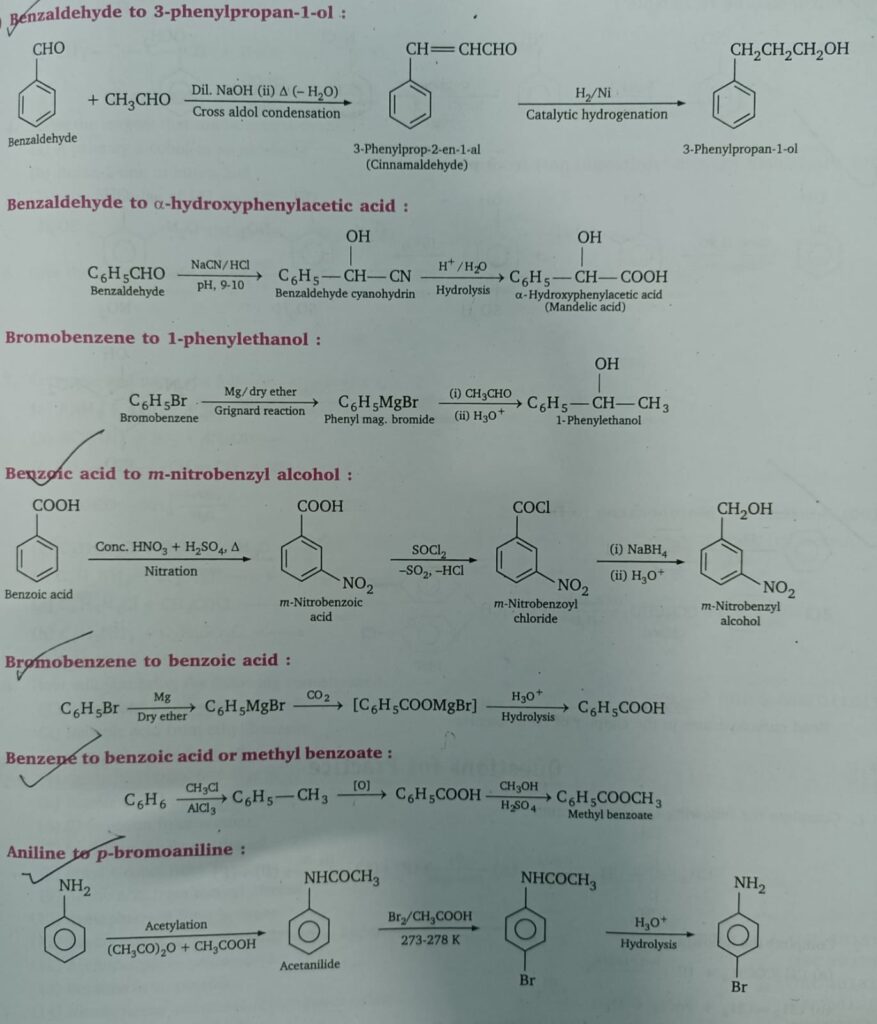
- Benzaldehyde to 3-phenylpropan-1-ol
- Benzaldehyde to Mandelic acid
- Bromobenzene to 1-phenylethanol
- Benzoic acid to m-nitrobenzyl alcohol
- Bromobenzene to benzoic acid
- Benzene to methyl benzoate
- Aniline to p-bromoaniline
- Benzamide to toluene
- Benzoic acid to phenol
- Phenol to acetophenone
- Benzene to benzophenone
- Phenol to benzoic acid
- Aniline to p-hydroxyazobenzene
- Nitrobenzene to p-aminobenze
- Bromobenzene to m-nitrobenzoic acid
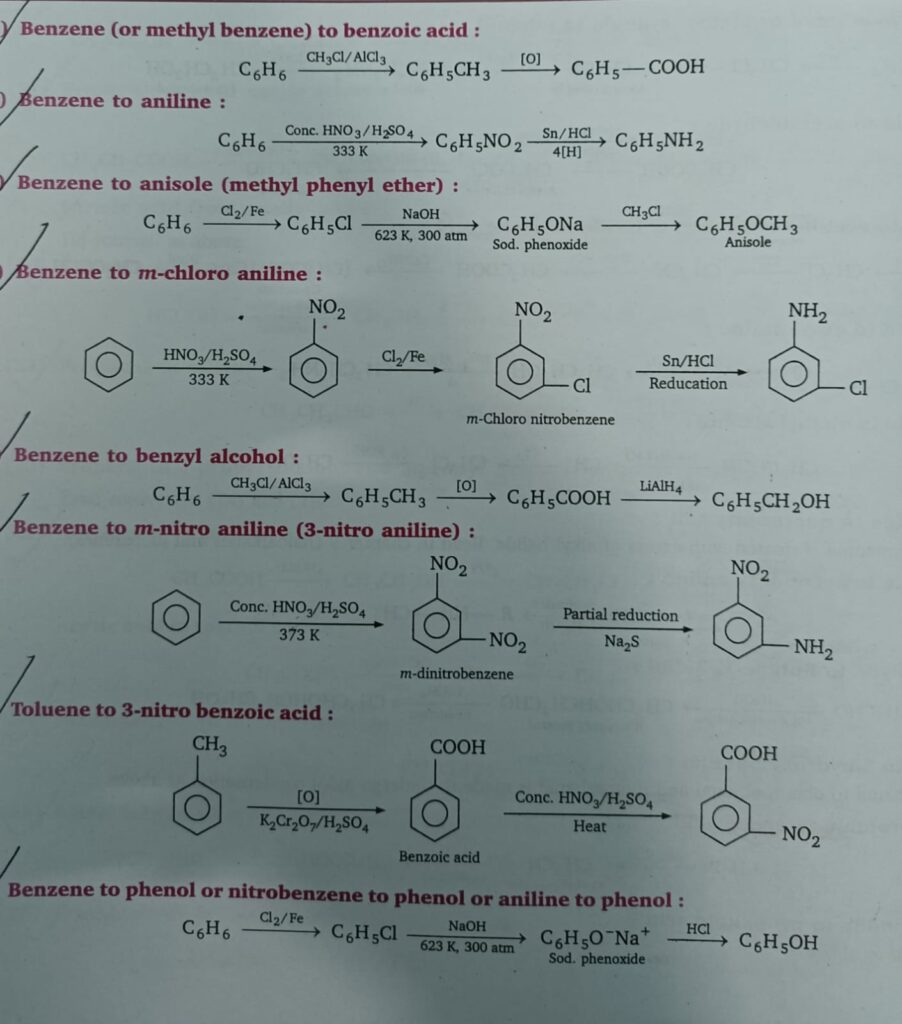
- Benzene to benzoic acid
- Benzene to aniline
- Benzene to anisole
- Benzene to m-chloro aniline
- Benzene to benzyl alcohol
- Benzene to m-nitro aniline
- Toluene to 3-nitro benzoic acid
- Benzene to phenol
- Ethanol to Propanone
- Ethyl alcohol to ethylene glycol
- Propan-2-ol to ethylene glycol
- Propan-2-ol to 1-bromopropane
- Ethyl alcohol to tertiary butyl alcohol
- n-Propyl alcohol to isopropyl alcohol
- isopropyl alcohol to n-propyl alcohol
- Acetylene to n-butyl alcohol
- Ethylene oxide to n-propyl alcohol
- n-butyl alcohol to dimethyl acetylene
- n-propyl alcohol to n-hexane
- Methyl alcohol to isopropyl alcohol
- Ethyl bromide to methane
- Ethyl alcohol to n-butyric acid
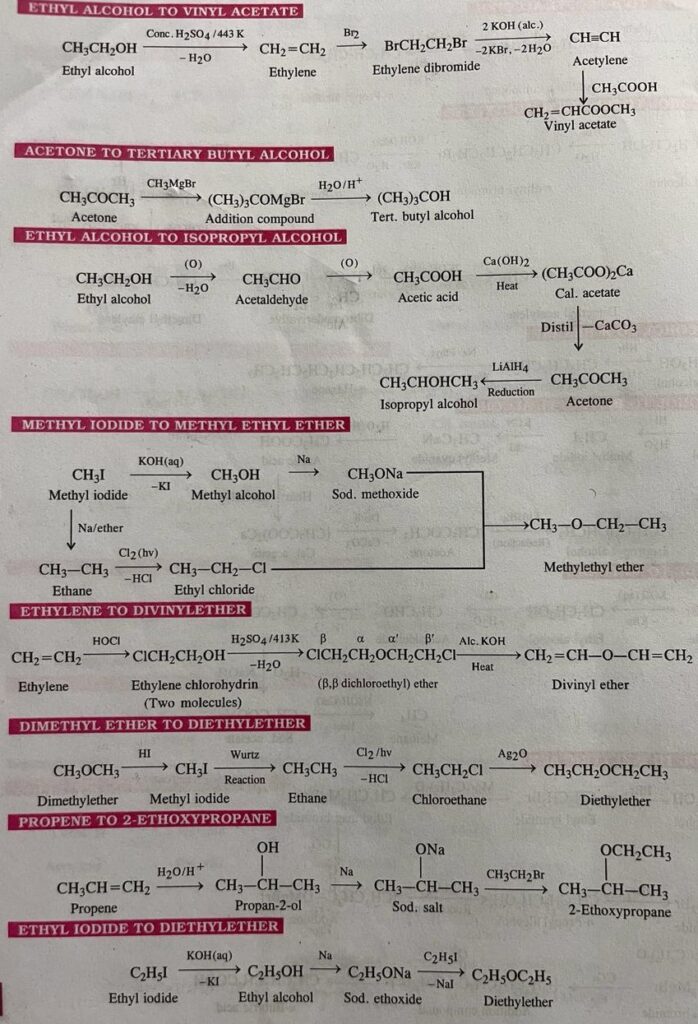
- Ethyl alcohol to vinyl acetate
- Acetone to tertiary butyl alcohol
- Ethyl alcohol to isopropyl alcohol
- Methyl iodide to methyl ethyl ether
- Ethylene to divinyl ether
- Dimethyl ether to diethyl ether
- Propene to 2-ethoxypropane
- Ethyl iodide to diethylether
- Ethanal to lactic acid
- Acetaldehyde to acetone
- Ethanal to ethyne
- Ethanal to 2-hydroxy but-3-enoic acid
- Formaldehyde to n-butane
- Ethanol to Butan-2-one
- Acetaldehyde to Crotonic acid
- Propanal to Propyne
- Acetone to Phorone
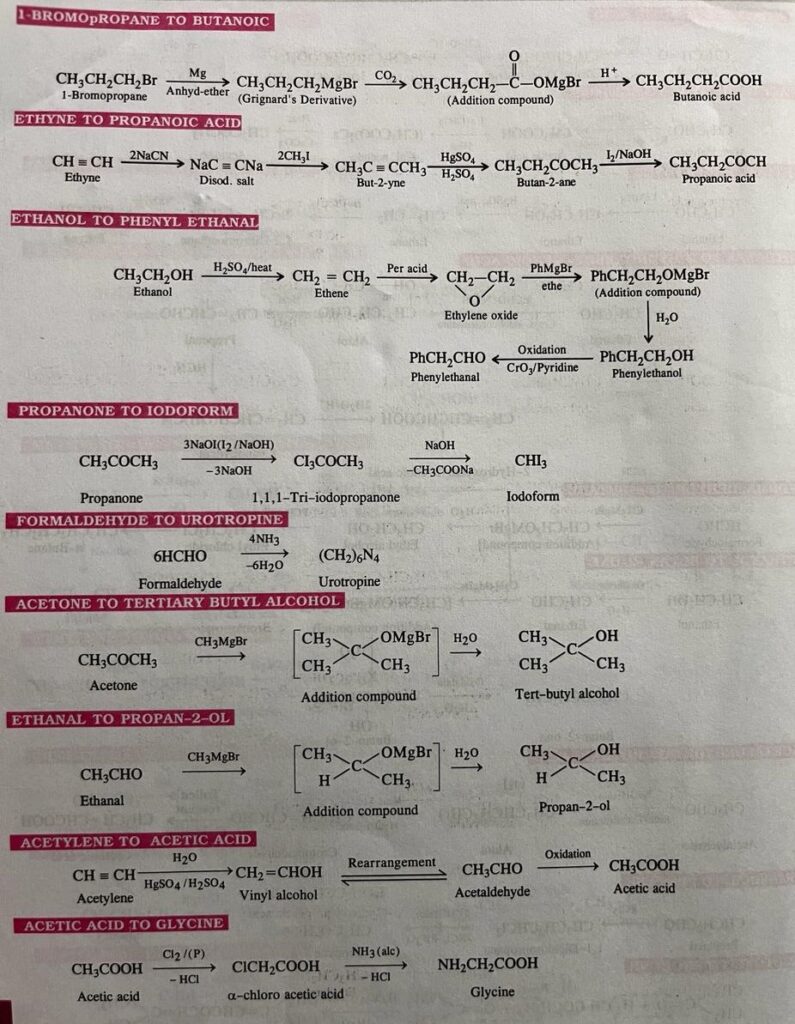
- 1-bromopropane to butanoic acid
- Ethyne to propanoic acid
- Ethanol to phenyl ethanal
- Propanone to iodoform
- Formaldehyde to urotropine
- Acetone to tertiary butyl alcohol
- Ethanal to propan-2-ol
- Acetylene to Acetic acid
- Acetic acid to Glycine
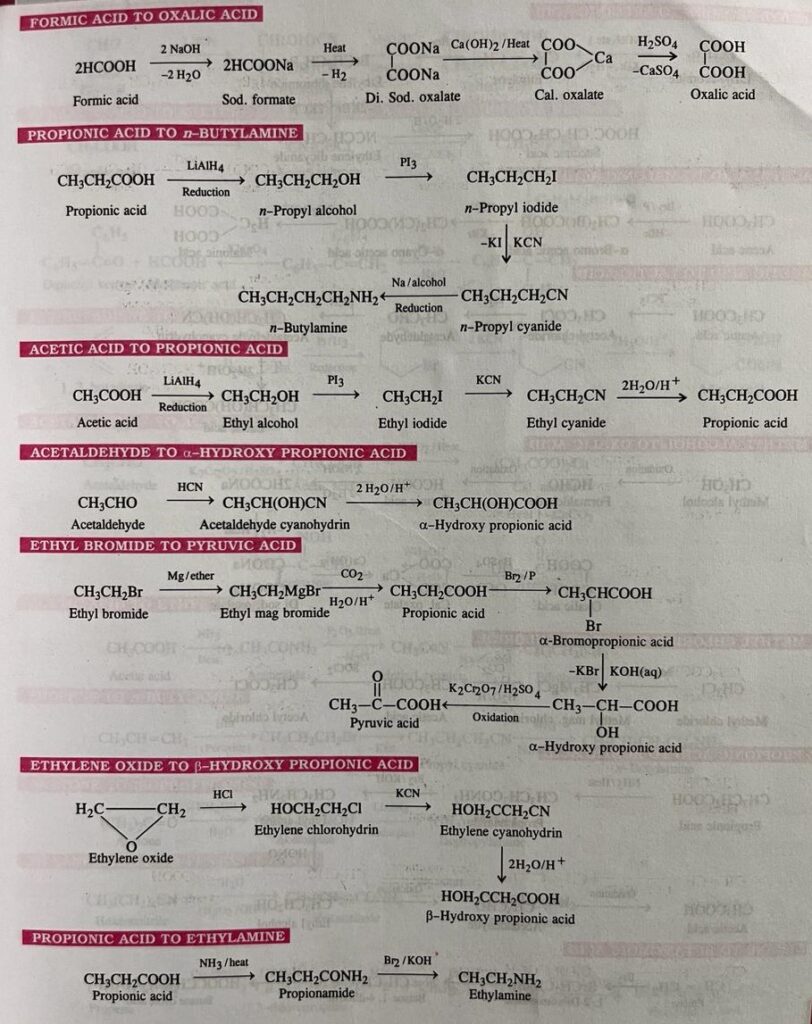
- Formic acid to oxalic acid
- Propionic acid to n-butylamine