In this post we have tried to give the students a complete writeup of Class 12 (ISC) Chemistry Practical.
Volumetric Analysis: Oxidation-Reduction Titration
A. Potassium manganate(VII) / ammonium iron (II) sulphate
1. Determination of water of crystallization of ammonium iron (II) sulphate.
You are provided with two solutions as follows:
C-10 is a solution containing 1.95 gms of potassium manganate(VII), KMnO4 per litre.
C-11 is a solution prepared by dissolving 23.2 gms of hydrated ammonium iron (II) sulphate crystals, (NH4)2SO4.FeSO4.xH2O per litre.
Procedure:
Rinse and fill the burette with the given solution C-10 (KMnO4). Pipette out 20 mL or 25 mL of C-11 (hydrated ammonium iron (II) sulphate solution) and transfer into a clean conical flask. To this add 20 mL of C-12 (dilute sulphuric acid) specially provided for titration.Titrate the solution in the conical flask with C-10 (KMnO4) slowly, till one drop of this gives a light permanent pink colour to the solution C-11 in the flask. The pink colour should not disappear on shaking the contents in the conical flask.Repeat the above procedure to get at least two concordant readings.Tabulate your readings.
State:
(a) The capacity of the pipette used.
(b) The titre value you intend to use in your calculations.
The equations for the above reactions are as follows:
2KMnO4 + 8H2SO4 + 10 (NH4)2SO4.FeSO4.xH2O → K2SO4 + 2MnSO4 +10(NH4)2SO4 + 5Fe2(SO4)3 + 8H2O + 10 x H2O
The ionic equation for the reaction is:
2MnO4– + 10Fe2+ + 16H+ → 2Mn2+ + 10Fe3+ + 8H2O
[K=39, Fe= 56, Mn= 55, S=32, N=14, H=1, O=16]
Calculate the following:
- The molarity of the solution of Potassium manganate(VII) C-10.
- The molarity of hydrated ammonium iron(II) sulphate solution C-11.
- The molecular mass of hydrated ammonium iron(II) sulphate deduced from the experimental data.
- The numerical value of x in (NH4)2SO4.FeSO4.xH2O.
Answer:


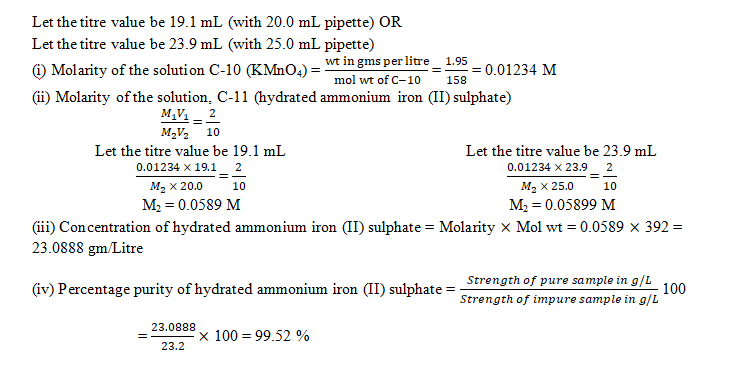
2. Determination of percentage purity of ammonium iron (II) sulphate in the impure sample
You are provided with two solutions as follows:
C-10 is a solution containing 1.95 gms of potassium manganate(VII), KMnO4 per litre.
C-11 is a solution prepared by dissolving 23.2 gms of impure sample of hydrated ammonium iron (II) sulphate per litre.
Procedure:
Rinse and fill the burette with the given solution C-10 (KMnO4). Pipette out 20 mL or 25 mL of C-11 (hydrated ammonium iron (II) sulphate solution) and transfer into a clean conical flask. To this add 20 mL of C-12 (dilute sulphuric acid) specially provided for titration.Titrate the solution in the conical flask with C-10 (KMnO4) slowly, till one drop of this gives a light permanent pink colour to the solution C-11 in the flask. The pink colour should not disappear on shaking the contents in the conical flask.Repeat the above procedure to get at least two concordant readings.Tabulate your readings.
State:
(a) The capacity of the pipette used.
(b) The titre value you intend to use in your calculations.
The equations for the above reactions are as follows:
2KMnO4 + 8H2SO4 + 10 (NH4)2SO4.FeSO4.xH2O → K2SO4 + 2MnSO4 +10(NH4)2SO4 + 5Fe2(SO4)3 + 8H2O + 10 x H2O
The ionic equation for the reaction is:
2MnO4– + 10Fe2+ + 16H+ → 2Mn2+ + 10Fe3+ + 8H2O
[K=39, Fe= 56, Mn= 55, S=32, N=14, H=1, O=16]
Calculate the following:
- The molarity of the solution of Potassium manganate(VII) C-10.
- The molarity of hydrated ammonium iron (II) sulphate solution C-11.
- The concentration of hydrated ammonium iron (II) sulphate in gm/litre.
- The percentage purity of hydrated ammonium iron (II) sulphate in the impure sample.
Answer:
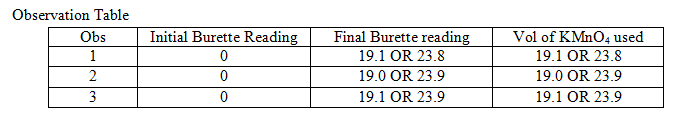
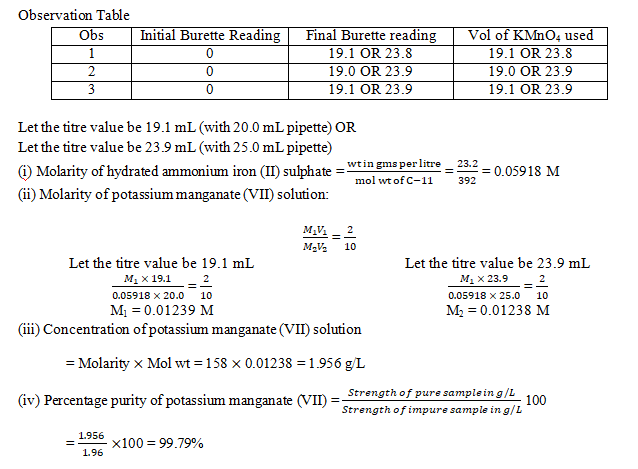
3.Determination of percentage purity of impure sample of potassium manganate (VII) in the impure sample.
You are provided with two solutions as follows:
C-10 is a solution containing 1.96 gms of potassium manganate (VII), KMnO4 per litre.
C-11 is a solution prepared by dissolving 23.2 gms of impure sample of hydrated ammonium iron (II) sulphate per litre.
Procedure:
Rinse and fill the burette with the given solution C-10 (KMnO4). Pipette out 20 mL or 25 mL of C-11 (hydrated ammonium iron (II) sulphate solution) and transfer into a clean conical flask. To this add 20 mL of C-12 (dilute sulphuric acid) specially provided for titration.Titrate the solution in the conical flask with C-10 (KMnO4) slowly, till one drop of this gives a light permanent pink colour to the solution C-11 in the flask. The pink colour should not disappear on shaking the contents in the conical flask.Repeat the above procedure to get at least two concordant readings.Tabulate your readings.
State:
(a) The capacity of the pipette used.
(b) The titre value you intend to use in your calculations.
The equations for the above reactions are as follows:
2KMnO4 + 8H2SO4 + 10 (NH4)2SO4.FeSO4.xH2O → K2SO4 + 2MnSO4 +10(NH4)2SO4 + 5Fe2(SO4)3 + 8H2O + 10 x H2O
The ionic equation for the reaction is:
2MnO4– + 10Fe2+ + 16H+ → 2Mn2+ + 10Fe3+ + 8H2O
[K=39, Fe= 56, Mn= 55, S=32, N=14, H=1, O=16]
Calculate the following:
- The molarity of the solution of hydrated ammonium iron (II) sulphate C-11.
- The molarity of potassium manganate (VII) solution C-10.
- The concentration of potassium manganate (VII) solution C-10.
- The percentage purity of potassium manganate (VII) in the impure sample.
Answer:
B. Potassium manganate(VII) / oxalic acid
1. Determination of the percentage purity of sample of oxalic acid solution.
You are provided with two solutions as follows:
C-10 is a solution containing 2.8 gms of potassium manganate (VII), KMnO4 per litre.
C-11 is a solution prepared by dissolving 6.25 gms of impure sample of oxalic acid crystals (H2C2O4.2H2O) per litre.
Procedure:
Rinse and fill the burette with potassium manganate (VII) solution C-10 (KMnO4). Pipette out 20 mL or 25 mL of the oxalic acid solution C-11 (H2C2O4.2H2O) in a clean conical flask. To this, add 20 mL of dilute sulphuric acid (H2SO4) C-12, specially provided for this purpose. Warm the contents of the flask to 60oC -70oC. The heating should be continued till the first bubble appears at the bottom of the flask. Remove the conical flask from fire and titrate this solution by running solution C-10 from the burette. Shake the solution constantly till a permanent pale pink colour is obtained. Ensure that the pink colour obtained does not disappear on shaking the contents of the conical flask. Repeat the above procedure to get at least two concordant readings. Tabulate your readings.
State:
(a) The capacity of the pipette used.
(b) The titre value you intend to use in your calculations.
The equations for the above reactions are as follows:
2KMnO4 + 4H2SO4 + 5H2C2O4 → K2SO4 + 2MnSO4 + 8H2O + 10CO2
2MnO4– + 5C2O42- + 16 H+ → 2Mn2+ + 10CO2 + 8H2O
[K=39, Mn=55, C=12, O=12, H=1]
Calculate the following:
- The molarity of potassium manganate (VII) solution C-10.
- The molarity of oxalic acid solution C-11.
- The strength of oxalic acid solution in gms per litre.
- The percentage purity of the sample of oxalic acid solution.
Answer:
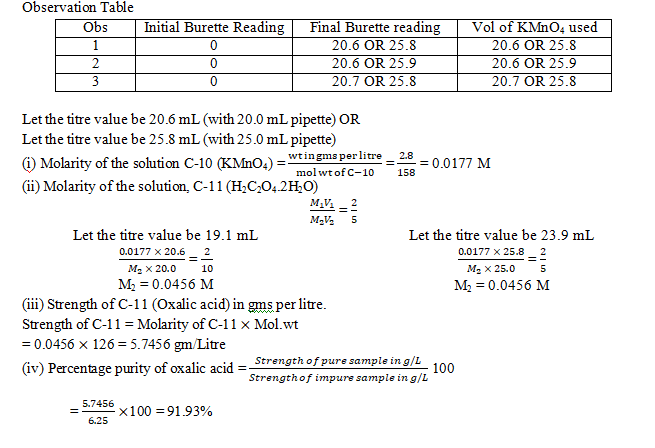
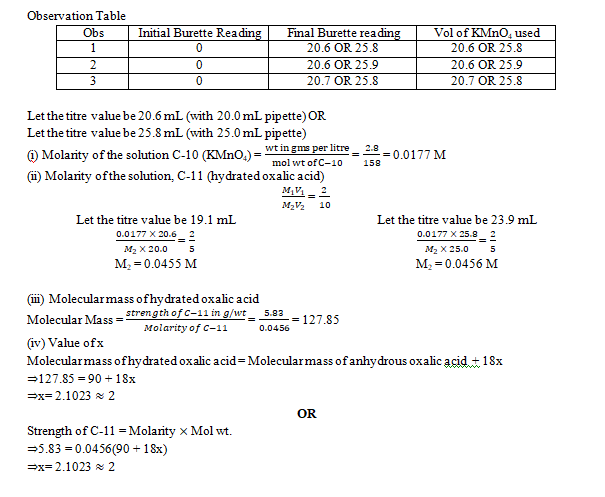
2.Determination of water of crystallization of oxalic acid.
You are provided with two solutions as follows:
C-10 is a solution containing 2.8 gms of potassium manganate (VII), KMnO4 per litre.
C-11 is a solution prepared by dissolving 5.83 gms of impure sample of oxalic acid crystals (H2C2O4.2H2O) per litre.
Procedure:
Rinse and fill the burette with potassium manganate (VII) solution C-10 (KMnO4). Pipette out 20 mL or 25 mL of the oxalic acid solution C-11 (H2C2O4.2H2O) in a clean conical flask. To this, add 20 mL of dilute sulphuric acid (H2SO4) C-12, specially provided for this purpose. Warm the contents of the flask to 60oC -70oC. The heating should be continued till the first bubble appears at the bottom of the flask. Remove the conical flask from fire and titrate this solution by running solution C-10 from the burette. Shake the solution constantly till a permanent pale pink colour is obtained. Ensure that the pink colour obtained does not disappear on shaking the contents of the conical flask. Repeat the above procedure to get at least two concordant readings. Tabulate your readings.
State:
(a) The capacity of the pipette used.
(b) The titre value you intend to use in your calculations.
The equations for the above reactions are as follows:
2KMnO4 + 4H2SO4 + 5H2C2O4 → K2SO4 + 2MnSO4 + 8H2O + 10CO2
2MnO4– + 5C2O42- + 16 H+ → 2Mn2+ + 10CO2 + 8H2O
[K=39, Mn=55, C=12, O=12, H=1]
Calculate the following:
- The molarity of the solution of Potassium manganate (VII) C-10.
- The molarity of hydrated ammonium oxalic acid solution C-11.
- The molecular mass of hydrated oxalic acid deduced from the experimental data.
- The numerical value of x in H2C2O4.xH2O
Answer:
Qualitative Analysis
1. Dry Test for anions:
a) Dilute sulphuric acid is added to dry solid sample.

b) Concentrated sulphuric acid is added to dry solid sample and heated.
2. Preparation of sodium carbonate extract:
Dry sample and anhydrous sodium carbonate is taken in 1:3 ratio in a beaker and boiled with 20 mL of water for 10 to 15 mins. Filtered hot and with the filtrate(sodium carbonate extract S.E) the following test are done.
3. Wet test for anions.
| Experiment | Observation | Inference |
| Little amount of the S.E is taken in a test tube. Then few drops of sodium nitroprusside solution is added. | Violet colouration formed. | S2‑ confirmed |
| Dilute HCl is added to the S.E till effervescence ceases. Then BaCl2 solution is added. | White ppt formed which is soluble in dil. HCl. White ppt formed which is insoluble in dil. HCl. | SO32- confirmed SO42- confirmed |
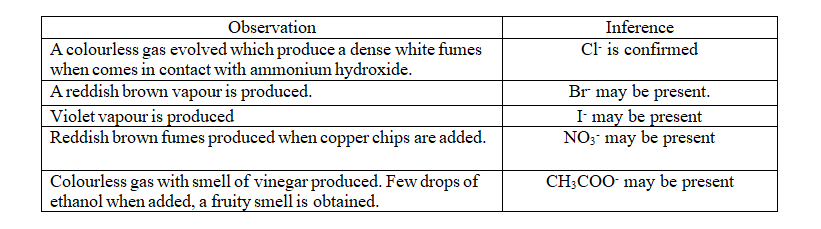
| Dilute HNO3 is added to the S.E till effervescence ceases. Then AgNO3 solution is added. | White ppt obtained which is soluble in NH4OH solution. | Cl– confirmed |
| Dilute HNO3 is added to the S.E till effervescence ceases. Then AgNO3 solution is added. | Pale yellow ppt obtained which is soluble in excess NH4OH solution. | Br– confirmed |
| Dilute HNO3 is added to the S.E till effervescence ceases. Then AgNO3 solution is added. | Yellow ppt obtained which is insoluble in NH4OH solution. | I– confirmed |
| Few drops of dlute sulphuric acid is added to the S.E followed by few drops of KI solution and freshly prepared starch solution. | The solution turns deep blue. | NO2– confirmed |
| To the S.E dilute sulphuric acid is added till effervescence ceases. Then freshly prepared ferrous sulphate is added. The mixture is cooled under running tap water. Then concentrated sulphuric acid is slowly added along the side of the test tube. | A dark brown ring is formed at the junction of the two liquids. | NO3– confirmed |
| To the S.E, few drops of neutral ferric chloride is added. | Red colour solution is formed which produce a brown ppt when heated. | CH3COO– confirmed |
| To the S.E few drops of dilute acetic acid is added followed by calcium chloride solution. | White ppt formed. | C2O42- confirmed |
| To the S.E concentrated nitric acid is added and warmed with excess ammonium molybdate solution. | Canary yellow ppt formed. | PO43‑ confirmed. |
4. Preparation of Original solution:
| Cold water | Hot water | Dil HCl | Conc.HCl | Aqua Regia |
The sample given is soluble in ______ to produce the original solution.
5. Group Analysis:
| Experiment | Observation | Inference |
| Dil. HCl is added to the original solution. | White ppt formed. | Group I present |
| Filtered. H2S is passed through the filtrate. | Black ppt formed. | Group II present. |
| Filtered. H2S is boiled off. Then few drops of conc. HNO3 is added and reheated. NH4Cl is added followed by NH4OH till ammoniacal. | Ppt formed. | Group III present. |
| Filtered. H2S is passed through the filtrate. | Ppt formed. | Group IV present. |
| Filtered. The filtrate is heated to 1/3 of its mass. Then NH4Cl, NH4OH and saturated (NH4)2CO3 is added. | White ppt formed. | Group V present. |
| Filtered. Saturated Na2HPO4 is added to the filtrate. | White crystalline ppt formed. | Group VI present. Mg2+ confirmed. |
6. Confirmatory test for cations:
i) The residue of group I is dissolved in hot water and potassium chromate solution is added. Heavy yellow ppt is formed. Pb2+ confirmed.
ii) The residue of group II is dissolved in conc. HNO3 and then divided into two portions.
a) First portion is treated with potassium chromate solution. Heavy yellow ppt is formed. Pb2+ confirmed.
b) Second portion is treated with potassium ferrocyanide solution. Chocolate brown ppt formed. Cu2+ confirmed.
iii) The residue of group III is dissolved in conc. HCl and divided into two portions.
a) First portion is treated with sodium hydroxide solution. White gelatinous ppt obtained. Al3+ confirmed.
b) Second portion is treated with potassium ferrocyanide solution. Prussian blue colouration formed. Fe3+ confirmed.
iv) The residue of group IV is treated with minimum quantity of dil. HCl and heated for a long time to boil off H2S. Filtered.
Residue is dissolved in aqua regia or (NaOCl + HCl) and divided into two parts.
a) First portion is treated with amyl alcohol and shaken with NH4SCN. Amyl alcohol layer turns blue. Co2+ confirmed.
b) In the second portion NH4Cl is added followed by NH4OH till alkaline. Then excess of dimethyl glyoxime is added. Red ppt obtained. Ni2+ confirmed.
Filtrate is divided into two parts.
a) First part is treated with NaOH solution. The ppt formed is dissolved in conc. HNO3 in presence of PbO2. Purple colouration formed. Mn2+ confirmed.
b) In the second part potassium ferrocyanide solution is added. Bluish white ppt formed. Zn2+ confirmed.
v)The residue of group V is dissolved in dil.acetic acid and divided into three parts.
a) First part is treated with potassium chromate solution. Yellow ppt formed. Ba2+ confirmed.
b) If Ba2+ is absent in the first part, to the second portion ammonium sulphate solution is added. White ppt formed. Sr2+ confirmed.
c) If both Ba2+ and Sr2+ are absent, ammonium oxalate solutions is added to the third portion. White ppt formed. Ca2+ confirmed.
7. Detection of group zero:
Dry sample is NaOH solution is added and heated. Pungent smelling gas produced which produce dense white fumes when come in contact with a glass rod dipped in conc. HCl. Group Zero present.
The gas produced is passed through nessler’s reagent. The Nessler’s reagent turns brown. NH4+ confirmed.
Identification of organic compounds and functional groups based on observations
1. Alcoholic group- glycerol
| Experiment | Observation |
| a) To 3 mL of 1% borax solution taken in a clean test tube, few drops of phenolphthalein solution are added. To this solution, few drops of sample is added and shaken. | Pink colour gets discharged. |
| b) To 1 mL of the sample solution ,4-5 drops of phenol is added followed by 2-3 drops of conc. sulphuric acid. The mixture is heated. Cooled. Diluted with water and finally ammonium hydroxide solution is added. | A red coloured solution is produced |
| c) To 1 mL of the sample solution, 1 mL of copper sulphate solution is added and then 1 mL of sodium hydroxide solution is added. | A blue coloured solution is produced. |
2. Aldehyde group- formaldehyde
| Experiment | Observation |
| a) To 2 mL of the sample few crystals of resorcinol is added and shaken. Then 1 mL of conc. sulphuric acid is along the side of the test tube. | A red ring is formed at the junction of the two liquids while a white ppt forms in aqueous layer. |
| b) To 2 mL of the sample , 1 mL of freshly prepared pyrogallol solution is added. The content is shaken. 2 mL of conc. HCl is added and then the content is warmed in a water bath. | A white ppt is formed which readily turns pink and then deep red. |
| c) To 2 mL of the sample, 1 mL of tollen’s reagent is added and warmed in hot water bath. | A shiny silver mirror is formed. |
3. Ketonic group- acetone
| Experiment | Observation |
| a) Few crystals of iodine are taken and then 2 mL of the sample is added. The mixture is shaken to dissolve iodine. Few drops of NaOH solution is added and warmed. | Yellow ppt formed. |
| b) To 2 mL of the sample solution, 0.5 mL of NaOH solution is added followed by 2-3 drops of sodium nitroprusside solution. Allowed to stand for some time and then warmed gently. | A ruby red colouration formed which fades to yellow or disappears. |
| c) To about 0.5 mL of mercuric chloride solution, NaOH solution is added till no further change occurs. To it sample solution is added. | Yellow crystals formed dissolves on adding the sample solution. |
4.Carboxylic acid- benzoic acid
| Experiment | Observation |
| a) The sample solution is treated with moist blue litmus solution. | The solution turns red. |
| b) To 2 mL of the sample solution, 2 mL of sodium bicarbonate solution is added. | Effervescence seen. |
| c) To 2 mL of the sample solution, 2 mL of ethanol is added and few drops of conc. H2SO4 are added. The mixture is warmed. | A pleasant fruity smell is obtained. |
5. Amino group-aniline
| Experiment | Observation |
| a) To 1 mL of the sample solution, 1 mL of conc. HCl is added. Now, few drops of neutral ferric chloride solution is added and then diluted with water. | Pale green colouration. |
| b) To 1 mL of the sample solution 2-3 drops of sodium hypochlorite solution is added and shaken. | Violet or purple colouration is obtained. |
| c) To 1 mL of the sample solution few drops of dilute sulphuric acid is added followed by 1 mL of potassium dichromate solution. The mixture is shaken and warmed. | A deep red colouration is produced which finally changes to deep blue or black. |
Characteristic Tests of carbohydrates and proteins
1. Carbohydrate-glucose
| Experiment | Observation |
| a) To 2 mL of the sample solution, 1 mL of Fehling’s solution is added and warmed. | A brick red ppt is obtained. |
| b) To 2 mL of the sample solution, 2-3 drops of α-naphthol solution is added. After that, conc. sulphuric acid is slowly added along the side of the test tube. | A violet ring is formed at the junction of the two liquids. |
| c) To 1 mL of the sample solution, 1mL of lead acetate solution is added followed by ammonium hydroxide solution. The content is boiled. | A white ppt is formed which turns to salmon pink on boiling with ammonium hydroxide. |
2. Protein- powdered milk
| Experiment | Observation |
| a) The sample is dissolved in water. 2 mL of such solution is treated with 1 mL of copper sulphate solution and shaken. Now 3 mL of NaOH solution is added. | Violet colouration is developed. |
| b) The sample is dissolved in water. 2 mL of such solution is treated with 1 mL of nitric acid. The content is boiled. The 5 mL of ammonium hydroxide solution is added. | Orange colour is produced. |
Study of the rate of reaction
This experiment is designed to find the effect of concentration of the reactants on the rate of a chemical reaction.
You are provided with two solutions:
(a) C-13 is a solution of sodium thiosulphate crystals of strength 0.2 M
(b) C-14 is a solution of HCl of strength 0.1 M.
Procedure:
Take 5 beakers labelled 1 to 5. With the help of a measuring cylinder, put sodium thiosulphate solution and distilled water according to the table given below:
| Beaker No. | Volume of Solution C-13 | Volume of distilled water | Time in seconds |
| 1 | 50 mL | 0 mL | |
| 2 | 40 mL | 10 mL | |
| 3 | 30 mL | 20 mL | |
| 4 | 20 mL | 30 mL | |
| 5 | 10 mL | 40 mL |
Place beaker number 1 over a piece of paper with a cross mark (X) on it.
Now add 10 mL of the solution C-14(HCl) to this solution and start the stop-watch at the same time. Swirl the contents of the beaker and return it over the over mark.
Look down vertically on the cross mark and stop the stop-watch as soon as tge cross on the paper become invisible. Note the time in the stop-watch.
Na2S2O3 (aq) + 2HCl(aq) → 2NaCl (aq) + SO2(g) + H2O + S(Colloidal)
Repeat the experiment by adding 10 mL of C-14 to the beaker labelled 2,3,4 and 5 and note the time taken in each case for the cross mark on the paper to become invisible.
Tabulate the readings. Find your results:
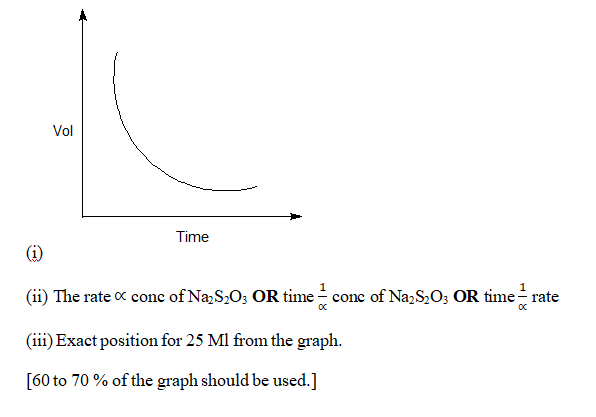
(i) Plot a graph on the concentration of sodium thiosulphate solution ( in terms of the volume of the sodium thiosulphate taken) against time taken for the cross mark to become invisible.
(ii) Predict the effect of change in concentration of sodium thiosulphate on the rate of the above reaction from the nature of your graph.
(iii) From the graph , find the time taken for the reaction when 25 mL of the solution C-13 is used.
Answer