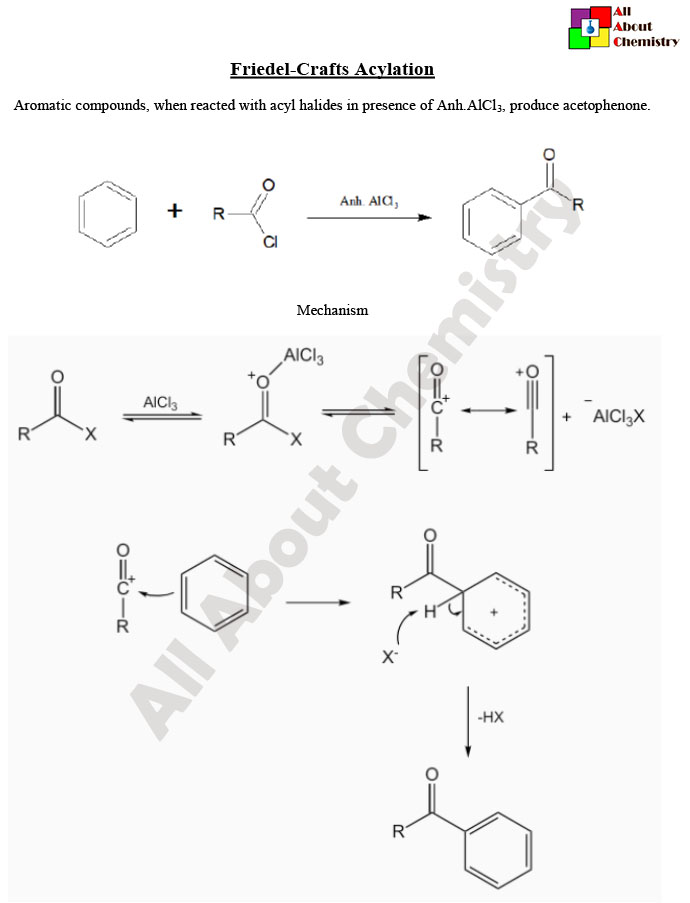
Friedel-Crafts acylation is a classic organic reaction used to add an acyl group (RCO-) to an aromatic ring. It’s named after the French chemist Charles Friedel and the American chemist James Crafts, who developed it in the late 19th century. The reaction involves the use of a Lewis acid catalyst, typically aluminum chloride (AlCl3) or ferric chloride (FeCl3), to facilitate the acylation process.
The mechanism typically involves several steps:
- Formation of Acyl Cation: The Lewis acid catalyst coordinates with the acid chloride (RCOCl), activating it by polarizing the C=O bond. This creates an acyl cation (RCO⁺) and a chloride ion (Cl⁻) as shown:
RCOCl+Lewis Acid→RCO+ + Cl−
- Electrophilic Aromatic Substitution (EAS): The activated acyl cation acts as an electrophile and reacts with the aromatic compound (Ar-H) through electrophilic aromatic substitution (EAS). The aromatic ring donates electron density to the positively charged carbon, leading to the formation of a sigma complex intermediate:
Ar-H+RCO+→Ar-CO-R+H+
- Rearrangement and Deprotonation: The sigma complex undergoes rearrangement, leading to the formation of the acylated aromatic product (Ar-CO-R). Finally, the Lewis acid catalyst deprotonates the product, regenerating the aromaticity of the ring and completing the reaction.
Friedel-Crafts acylation is a valuable tool in organic synthesis for the preparation of aromatic ketones and related compounds. It’s widely used in the synthesis of pharmaceuticals, fragrances, dyes, and other fine chemicals. Additionally, modifications and variations of the reaction conditions have been developed to improve selectivity, substrate scope, and reaction efficiency.
Friedel-Crafts acylation finds numerous applications in organic synthesis due to its ability to introduce acyl groups onto aromatic rings. Some notable applications include:
-
Pharmaceutical Synthesis: Friedel-Crafts acylation is widely used in the pharmaceutical industry for the synthesis of key intermediates and active pharmaceutical ingredients (APIs). Aromatic ketones produced via this reaction serve as essential building blocks in the synthesis of various drugs. For example, aromatic ketones can be further elaborated into non-steroidal anti-inflammatory drugs (NSAIDs), antihistamines, and other therapeutic agents.
-
Flavor and Fragrance Chemistry: Many flavor and fragrance molecules contain aromatic ketone functionalities. Friedel-Crafts acylation provides a route to these compounds, allowing for the synthesis of natural and synthetic flavoring agents, fragrances, and aroma chemicals. These compounds are essential in the food, beverage, cosmetics, and personal care industries.
-
Polymer Chemistry: Aromatic ketones synthesized via Friedel-Crafts acylation can be utilized in polymerization reactions. They can serve as monomers for the preparation of aromatic ketone-based polymers, such as poly(aryl ketone)s. These polymers exhibit high thermal stability, chemical resistance, and mechanical strength, making them valuable in applications requiring durable materials, such as aerospace, automotive, and electronics industries.
-
Material Science: Aromatic ketones derived from Friedel-Crafts acylation are employed in the synthesis of various functional materials. They can be utilized in the preparation of liquid crystals, organic light-emitting diodes (OLEDs), and organic semiconductors. These materials have applications in display technologies, optoelectronic devices, and organic electronics.
-
Natural Product Synthesis: Friedel-Crafts acylation is used in the synthesis of natural products and their analogs. By introducing acyl groups onto aromatic rings, chemists can access key intermediates for the total synthesis of complex natural products. This approach enables the study of structure-activity relationships and the development of novel bioactive compounds.
-
Fine Chemical Synthesis: The reaction is valuable in the synthesis of specialty chemicals, agrochemicals, and other fine chemicals. Aromatic ketones produced via Friedel-Crafts acylation serve as versatile intermediates for the preparation of diverse compounds with specific functional groups and properties.
Overall, Friedel-Crafts acylation is a versatile and widely applied reaction in organic synthesis, playing a crucial role in the preparation of a broad range of compounds with varied applications in pharmaceuticals, flavor and fragrance chemistry, polymer science, material science, natural product synthesis, and fine chemical synthesis.