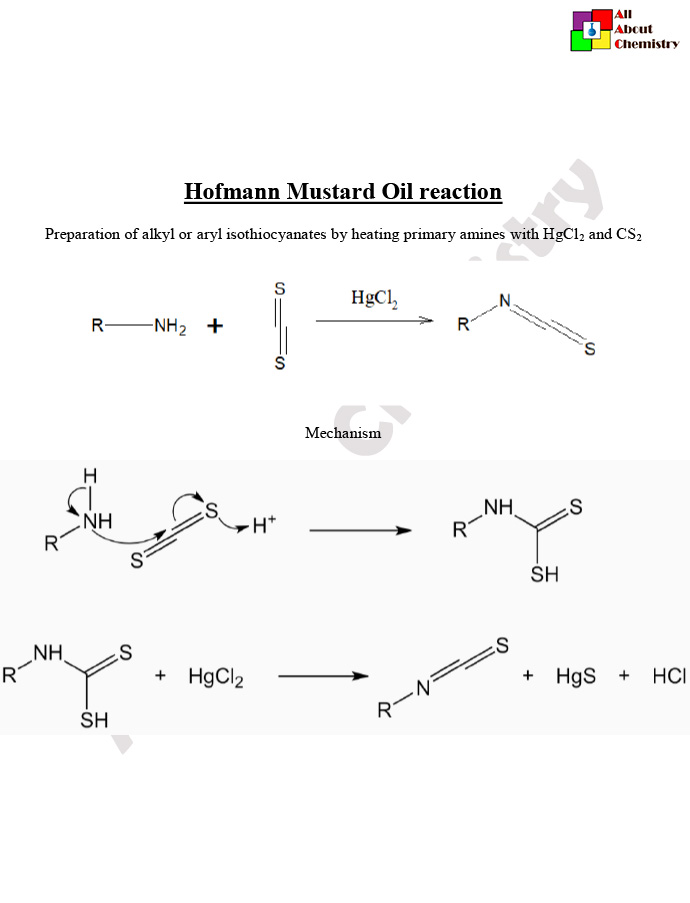
he Hofmann Mustard Oil reaction is a chemical transformation named after its discovery by August Wilhelm von Hofmann. This reaction involves the conversion of primary amides to isothiocyanates, which are also known as mustard oils. The reaction proceeds through a reaction with carbon disulfide (CS2) followed by hydrolysis under basic conditions. The general reaction scheme is as follows:
RCONH2+CS2+2NaOH→R−NCS+Na2CS3+2H2O
Here, R represents an organic substituent.
The mechanism of the Hofmann Mustard Oil reaction involves several steps. Here’s a simplified explanation:
- Formation of Dithiocarbamate Salt: The primary amide reacts with carbon disulfide (CS2) in the presence of a strong base (such as sodium hydroxide, NaOH) to form a dithiocarbamate salt. This step involves the nucleophilic attack of the amide nitrogen on the carbon atom of CS2.
RCONH2+CS2+2NaOH→R−NCSNa+Na2CS3+H2O
- Decomposition of Dithiocarbamate Salt: The dithiocarbamate salt formed in the first step undergoes decomposition, leading to the formation of the isothiocyanate (mustard oil) and sodium hydroxide.
R−NCSNa→R−NCS+NaOH
This step involves the cleavage of the C-N bond in the dithiocarbamate salt, leading to the liberation of the isothiocyanate product. The exact mechanism of this decomposition step may vary depending on the reaction conditions and the nature of the substrate.
Overall, the Hofmann Mustard Oil reaction involves the conversion of a primary amide to an isothiocyanate through the intermediacy of a dithiocarbamate salt. This reaction is widely used in organic synthesis for the preparation of isothiocyanates, which are valuable synthetic intermediates with various applications in organic chemistry, medicinal chemistry, and materials science.
The Hofmann Mustard Oil reaction, which converts primary amides to isothiocyanates, has several important applications in organic synthesis, medicinal chemistry, and materials science:
- Synthesis of Isothiocyanates: The primary application of the Hofmann Mustard Oil reaction is in the synthesis of isothiocyanates (mustard oils) from primary amides. Isothiocyanates are versatile building blocks in organic synthesis due to their ability to react with a variety of nucleophiles, such as amines and thiols, to form a wide range of compounds. They are used in the synthesis of pharmaceuticals, agrochemicals, dyes, and materials.
- Synthetic Intermediates: Isothiocyanates obtained from the Hofmann Mustard Oil reaction serve as valuable intermediates in the synthesis of various compounds. They can undergo reactions such as nucleophilic addition, cycloaddition, and substitution to yield functionalized products. These reactions enable the introduction of diverse functional groups and the construction of complex molecular structures.
- Medicinal Chemistry: Isothiocyanates exhibit diverse biological activities and have been extensively studied for their potential pharmacological properties. They have shown promising anticancer, antimicrobial, anti-inflammatory, and antioxidant activities. The Hofmann Mustard Oil reaction provides a convenient method for the synthesis of isothiocyanates for biological evaluation and drug discovery efforts.
- Natural Products Synthesis: Isothiocyanates are found in various natural products, including plants of the Brassicaceae family (such as mustard, horseradish, and broccoli) and marine organisms. The Hofmann Mustard Oil reaction can be utilized in the synthesis of isothiocyanate-containing natural products and analogs for biological and pharmacological studies.
- Materials Science: Isothiocyanates have applications in materials science, particularly in the preparation of functionalized surfaces, polymers, and nanomaterials. They can be used as reactive intermediates for surface modification, polymer functionalization, and the preparation of self-assembled monolayers. The Hofmann Mustard Oil reaction provides a route for the incorporation of isothiocyanate functionalities into materials for various applications, including sensors, coatings, and biomaterials.
Overall, the Hofmann Mustard Oil reaction is a versatile synthetic tool for the preparation of isothiocyanates with applications spanning organic synthesis, medicinal chemistry, materials science, and natural product synthesis. Its broad utility makes it a valuable transformation in chemical research and development.










