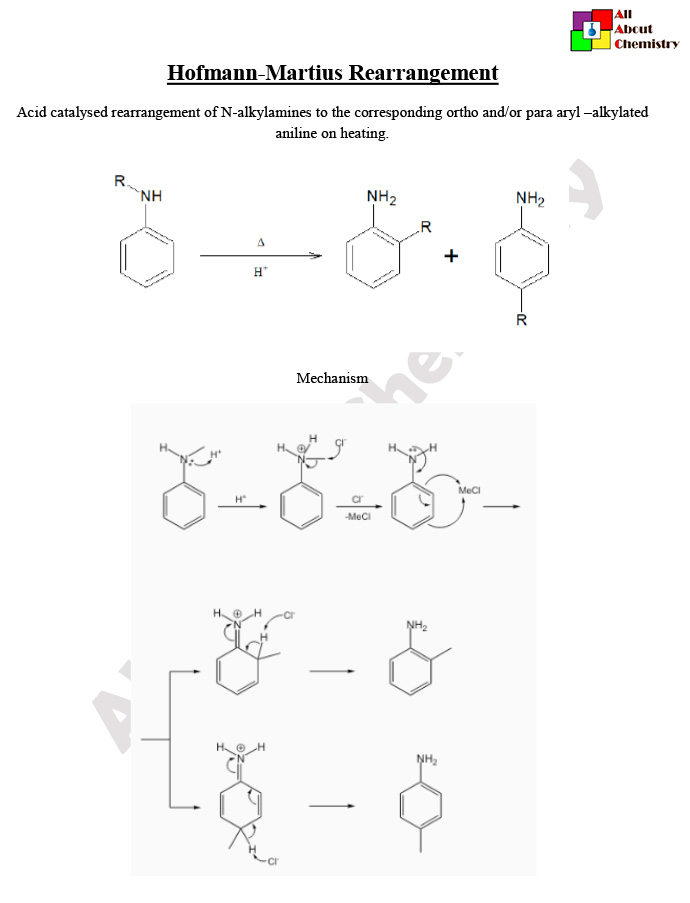
he Hofmann-Martius rearrangement is a chemical reaction named after its discoverers, August Wilhelm von Hofmann and Friedrich Martius. It involves the conversion of an N-aryl hydroxamic acid to an isocyanate. The general reaction scheme is as follows:

Here, Ar represents an aryl group. The reaction is usually carried out using bromine and a strong base such as sodium hydroxide in an aqueous or alcoholic solution. The mechanism involves the formation of an intermediate isocyanate via elimination of nitrogen. The isocyanate product can then undergo various reactions including the formation of carbamates or ureas.
The mechanism of the Hofmann-Martius rearrangement involves several steps. Here’s a simplified explanation:
- Protonation of Hydroxamic Acid: The reaction begins with the protonation of the hydroxamic acid (N-aryl hydroxamic acid) by a strong acid, typically hydrochloric acid (HCl). This protonation facilitates the subsequent steps by making the nitrogen more susceptible to nucleophilic attack.
- Formation of Oxoammonium Ion: The protonated hydroxamic acid undergoes intramolecular cyclization to form an intermediate called the oxoammonium ion. This intermediate is stabilized by resonance.
- Migration of Aryl Group: The lone pair of electrons on the nitrogen atom of the oxoammonium ion attacks one of the adjacent carbon atoms in the aryl ring, causing a migration of the aryl group from nitrogen to the adjacent carbon. This migration step is facilitated by the expulsion of a leaving group, typically a halogen ion (e.g., bromide).
- Formation of Isocyanate: The migration of the aryl group leads to the formation of an intermediate isocyanate. This intermediate is unstable and rapidly undergoes rearrangement to yield the final product, an aryl isocyanate.
Overall, the Hofmann-Martius rearrangement converts an N-aryl hydroxamic acid into an aryl isocyanate via a series of acid-catalyzed cyclization and rearrangement steps. The reaction is often carried out under basic conditions, where the base serves to neutralize the acid produced during the reaction and promote the elimination step.
The Hofmann-Martius rearrangement has several important applications in organic synthesis, particularly in the preparation of isocyanates, which are versatile intermediates in various chemical transformations. Some of the key applications include:
- Synthesis of Isocyanates: The primary application of the Hofmann-Martius rearrangement is in the synthesis of isocyanates. Isocyanates are essential building blocks for the preparation of polyurethanes, which are widely used in the production of foams, elastomers, coatings, adhesives, and sealants. The rearrangement provides a convenient and efficient method for the preparation of aryl isocyanates from N-aryl hydroxamic acids.
- Synthesis of Ureas and Carbamates: Isocyanates obtained from the Hofmann-Martius rearrangement can be used in the synthesis of ureas and carbamates through reactions with amines and alcohols, respectively. Ureas and carbamates are important functional groups in pharmaceuticals, agrochemicals, and materials science.
- Modification of Peptides: The Hofmann-Martius rearrangement has been utilized in peptide chemistry for the modification of peptides containing hydroxamic acid functionalities. This reaction allows for the introduction of various functional groups at specific positions within peptide sequences, enabling the synthesis of peptide analogs with altered properties or biological activities.
- Functionalization of Aromatic Compounds: The aryl isocyanates produced by the Hofmann-Martius rearrangement can undergo further functionalization reactions, such as nucleophilic addition reactions with nucleophiles like amines, alcohols, and thiols. This enables the synthesis of a diverse range of functionalized aromatic compounds with tailored properties for applications in materials science and medicinal chemistry.
- Synthesis of Biologically Active Compounds: The Hofmann-Martius rearrangement has been employed in the synthesis of biologically active compounds, including pharmaceuticals and natural products. Isocyanates and their derivatives play crucial roles in the development of pharmaceutical agents targeting various diseases, such as cancer, inflammation, and infectious diseases.
Overall, the Hofmann-Martius rearrangement offers synthetic chemists a powerful tool for the preparation of diverse compounds with applications spanning materials science, pharmaceuticals, and agrochemicals. Its versatility and efficiency make it a valuable transformation in organic synthesis.










