Water is an essential for all life in earth. Although 71% of this earth contains water, only 2.5% of the total is fresh. And only 1% of the available fresh water is fit for human consumption. Previously water was regarded as an element. In 1781 Cavendish first proved that it was a chemically compound. The volumetric and gravimetric composition of water are 2:1 and 1:8 (H:O) respectively.
Structure:
Water exists in three phases in nature- solid(ice), liquid (water) and gas (steam or water vapour).
In a water molecule, oxygen atom remains in sp3 hybridised state. Two of these sp3 hybridised orbitals are overlap with
1s-orbital of hydrogen to form two bonds. While the other two sp3 hybridised orbitals contain a lone pair of electrons each.Due to the presence of lone pair of electrons, the H-O-H bond angle gets distorted from the regular tetrahedral value. In water the H-O-H angle is 104.50, O-H bond length is 95.7 pm and the shape is called bent shape.
The O-H bonds are polar where O atom carries a partial negative charge and each of the H atoms carries a partial positive charge due to large electronegativity difference between O (3.5) and H(2.1). The dipole moment of water is 1.84D.
Structure of water in gas phase:
In gaseous phase, water exist as isolated molecules with a bent structure. (Bond angle 104.50 and bond length 95.7 pm).
Structure of water in liquid phase:
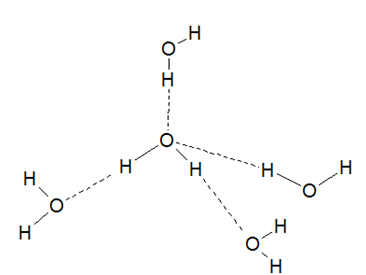
In the liquid state, water exists as associated molecules. Water molecules are held together by intermolecular hydrogen bonds in the liquid phase. Experimental studies suggest that each water molecule remains attached to four other water molecules.
Structure of water in solid phase:
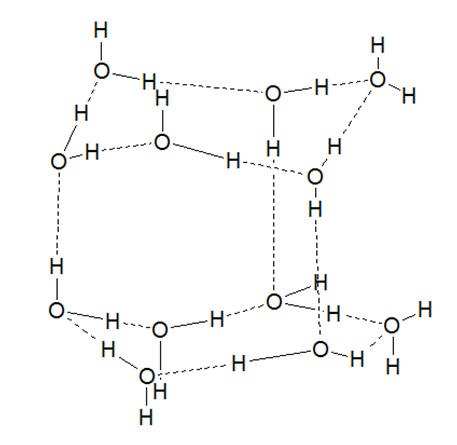
In solid phase (ice), water can exist in nine crystaline forms depending upon the conditions for freezing. At normal atmospheric pressure , ice crystalline in normal hexagonal form( ice 1h) but at very low temperature , it condenses in the cubic form( ice 1c).In the normal hexagonal ice, each water molecule remain surrounded by four other water molecules joined together by hydrogen bond to give a cage like structure.As hydrogen bonds are longer than covalent bonds, the water molecules are not tightly packed in the crystalline lattice. Due to the availability of vacant spaces in the crystalline lattice, the density of ice is lower than that of water. This explains why ice floats on water. As ice melts, a number of hydrogen bonds break and the water molecules come close to each other filling up the vacant spaces causing increase in the density. At 40C the water molecules remain at the closest approximity to each other.That is why water shows maximum density(1g/cc) at 40C. As the temperature exceeds, the water molecules starts dispersing from each other (due increased kinetic energy of the water molecules) causing decreasing in density( or increase in volume).This unusual behavior of water is known as anomalous expansion of water.
Properties of water:
Physical properties:
Water shows many unique properties due to the presence of hydrogen bond in between the molecules.
| Properties | H2O |
| Molar Mass | 18.0151 |
| Appearance | Colourless, transparent |
| Freezing point | 273.15 K |
| Boiling point | 373.15 K |
| Dipole moment | 1.84D |
| Density at STP (g/mL) | 0.9982 |
| Dielectric constant | 78.39 |
| Temp. of maximum density | 3.98 °C |
| Surface tension (at 25 °C, N/m) | 0.07198 |
| Heat of fusion (kJ/mol) | 6.00678 |
| Heat of formation (kJ/mol) | -285.9 |
| Heat of vaporisation (kJ/mol) | 40.657 |
| pH (at 25 °C) | 7.0 |
| Ionisation constant (mol2L-2) | 1.008 x 10-14 |
The importance of the various physical properties of water are-
- The refractive index of water increases with the increase in salinity and decreases with the increase in temperature.
- High latent heat of vaporisation of water helps to control the weather and climate of earth.
- High heat capacity of water prevents extreme range in Earth’s temperature.
- High surface tension of water helps to control formation of drop in rain and clouds.
- The anomalous expansion of water helps the marine life to sustain inside water in cold countries.
- High dielectric constant of water allows it to dissolve many substances in it. This property of water is used in different physical, chemical and biological processes.
Chemical Properties:
Amphoteric nature of water:
Water is a weak electrolyte. It undergoes ionization to a small extent to give H3O+ and OH– ions.
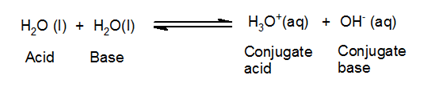
Water acts both as an acid and base.
- With HCl or H2S water acts as a base.
H2O + HCl →H3O+ + Cl–
Base Acid Acid Base
- With NH3 water acts as an acid.
H2O + NH3 →NH4+ + OH–
Acid Base Acid Base
As the degree of self-ionization of water is much lower, the electrical conductivity of pure water is very low.
As oxidising and reducing agent:
- When water reacts with electropositive elements like Na, K, Ca etc. it acts as an oxidizing agent to produce hydrogen.
2Na + 2H2O→2NaOH + H2
[2H2O + 2e–→2OH– + H2 ; E0 = -0.83V]
When steam is passed over red hot iron at 1273K, syngas is formed.
C +H2→CO + H2
In these reactions water act as an oxidising agent and hence itself gets reduced to hydrogen.
- When water reacts with fluorine,it acts as reducing agent to produce ozonised oxygen.
2F2 + 2H2O→O2+ 4HF
3F2 + 3H2O→O3+ 6HF
[O2+ 4H+ + 4e–→2H2O ; E0 = 1.23V]
During photosynthesis, water is oxidised to oxygen.

In these reactions water act as a reducing agent and hence itself get oxidised to produce oxygen.
Hydrolytic reaction:
Water can hydrolyse many metallic and non-metallic oxides, hydroxides, nitrides, carbides, phosphides and other salts producing acid, base or both.
CO2 + H2O→H2CO3 (Carbonic acid)
SO2 + H2O→H2SO3 (Sulphurous acid)
P4O10 + 6H2O→4H3PO4 (Phosphoric acid)
Na2O + H2O→2NaOH (Sodium hydroxide)
CaO + H2O→Ca(OH)2 (Calcium hydroxide)
CaH2 + 2H2O→Ca(OH)2 + 2H2
CaC2 + 2H2O→Ca(OH)2 + C2H2( Acetylene)
Al4C3 + 12H2O→4Al(OH)3 + 3CH4(Methane)
Ca3N2 + 6H2O→3Ca(OH)2 + 2NH3 (Ammonia)
AlN + 3H2O→Al(OH)3 + NH3
Ca3P2 + 6H2O→3Ca(OH)2 + 2PH3(Phosphine)
Na2CO3 + 2H2O→2NaOH + H2CO3
Hydrolysis of sodium carbonate produces a strong base (NaOH) and a weak base (H2CO3) , thus, an aqueous solution of sodium carbonate is alkaline in nature.
CuSO4 + 2H2O→Cu(OH)2 + H2SO4
Hydrolysis of copper sulphate produces a weak base Cu(OH)2 and a strong base (H2SO4) , thus, an aqueous solution of copper sulphate is acidic in nature.
Hydrate formation:
Certain salts contain water molecules in their molecules, such water molecules are called water of crystallisation and the crystals are called hydrated salts or hydrates.
Coordinated water: Water molecules which remain combined with the metal ion through coordinate bond to form complex ions are called coordinated water. For example, [Ni(OH2)6]2+SO42-, [Cr(OH2)6]3+3Cl–, [Fe(OH2)6]3+3Cl–etc.in all these compounds, the central metal ion remain coordinated to six water molecules.
Hydrogen bonded water: Water molecules that remain linked to an oxygen atom by hydrogen bond-forming oxo-anion are called hydrogen-bonded water. For example, in copper sulfate pentahydrated four water molecules are linked to the Cu2+ ion by the coordinate bond. While the fifth water molecule is linked to the sulfate ion by a hydrogen bond.
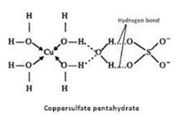
Interstitial water: Water molecules occupy the interstitial space or voids in the crystal lattice are called interstitial water. For example, in BaCl2.2H2O, two water molecules occupy the voids in the crystal lattice.
Solvent property:
Water is an excellent solvent-
- Water can stabilize ions by ion-dipole interactions due to its high polarity(μ=1.84D)
- Water can dissolve many ionic and covalent compounds due to its high dielectric constant (Є=78.39)(High ability to decrease the attractive force between oppositely charged ions).
- Water can dissolve many covalent compounds by forming a hydrogen bonds.
- Water molecules can donate and accept protons. Hence water can dissolve polar covalent compounds by acid-base reactions.
Due to such versatile solvent properties, water is called as a ‘universal solvent’.
Soft and hard water:
Water that lather easily with soap is called soft water. Example of soft water are- rain water, demineralised water and distilled water.Water that does not lather easily rather form a scum with soap is called hard water. Example of hard water are- sea, river, spring, lake and well water.Hardness of water is due to the presence of presence of sulphates, chlorides and bicarbonates of Mg and Ca.
When soap ( sodium salts of lauric acid(C12H23NaO2) , mristic acid(C14H27NaO2), palmitic acid(C16H31NaO2), stearic acid(C18H35NaO2), oleic acid(C18H33NaO2) and linoleic acid(C18H31NaO2) reacts with Ca2+ or Mg2+ scum or a curdy white precipitation is produced.
2CH3(CH2)16COONa + CaCl2→(CH3(CH2)16COO)2Ca + 2NaCl
Sodium stearate Calcium stearate
(Soap) (Curdy white ppt.)
Types of hardness of water:
Hardness of water is of two types-
Temporary hardness or carbonate hardness: Temporary hardness of water is due to the presence of bicarbonates of calcium and magnesium.
Permanent hardness or non-carbonate hardness: Permanent hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium.
Removal of hardness of water or softening of hard water:
The process of removal of Ca2+ and Mg2+ ions responsible for the hardness of water is known as softening of water. Depending upon the nature of dissolved salts, a number of methods are available to soften hard water.
1)Removal of temporary hardness: The temporary hardness of water can be removed by the following method:
a) Boiling process: When hard water is boiled, calcium and magnesium bicarbonates decompose to insoluble carbonates and precipitates out. These precipitations are removed by filtration.
Ca(HCO3)2 →CaCO3 ↓+ CO2 + H2O
Mg(HCO3)2→MgCO3 ↓+ CO2+ H2O
As MgCO3 is not completely insoluble in water, hence hardness caused by Mg(HCO3)2 cannot be completely removed by boiling.
b) Clark’s process: Calculated amount of slaked lime is added to hard water to remove calcium and magnesium in the form of carbonates and hydroxides.
Ca(HCO3)2 + Ca(OH)2→2CaCO3↓+ 2H2O
Mg(HCO3)2 + 2Ca(OH)2→2CaCO3↓+ Mg(OH)2↓+ 2H2O
Each mol of Mg(HCO3)2 requires 2mol of Ca(OH)2 to precipitate Mg(HCO3)2 completely as Mg(OH)2. If less than the requisite amount of Ca(OH)2 is taken, harness due to the magnesium will persist in water.
If more than the requisite amount of Ca(OH)2 is taken, hardness is created due to the absorption of atmospheric CO2 by Ca(OH)2 leading to the formation of Ca(HCO3)2 .
Ca(OH)2 + 2CO2→Ca(HCO3)2
Thus, calculated amount of slaked lime should be used in this process.
2) Removal of permanent hardness: Permanent hardness of water can be removed by washing soda process:
Washing soda process:
The chlorides and sulphates of magnesium and calcium are precipitated as insoluble carbonates when treated with calculated amount of washing soda(Na2CO3.10H2O).
MgSO4 + Na2CO3→MgCO3 ↓+ Na2SO4
Na2CO3→MgCO3 ↓+ 2NaCl
CaCl2 + Na2CO3→CaCO3 ↓+ 2NaCl
CaSO4 + Na2CO3→CaCO3 ↓+ Na2SO4
3) Removal of temporary and permanent hardness: The following methods can remove both temporary and permanent hardness of the water.
a) Soda lime method: In this method, the calculated amount of sodium carbonate (to remove permanent hardness) and slaked lime (to remove temporary hardness) is added to hard water.
Ca(HCO3)2 + Ca(OH)2→2CaCO3↓+ 2H2O
MgSO4 + Na2CO3→MgCO3↓+ Na2SO4
MgCl2 + Na2CO3→MgCO3↓+ 2NaCl
CaCl2 + Na2CO3→CaCO3↓+ 2NaCl
CaSO4 + Na2CO3→CaCO3↓+ Na2SO4
Little NaOH is added to facilitate the precipitation.
MgSO4 + 2NaOH→Mg(OH)2 ↓+ Na2SO4
Mg(HCO3)2 + 2NaOH→Mg(OH)2 ↓+ 2NaHCO3
b) Calgon method (sequestration): In this process, Ca2+ and Mg2+ ions present in hard water are rendered ineffective by treating with sodium hexametaphosphate. The trade name of sodium hexametaphosphate is Calgon.
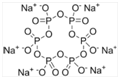
Calgon or sodium hexametaphosphate converts calcium and magnesium ions to soluble complexes which do not interact with soap.
2CaCl2 + Na2[Na4(PO3)6] → Na2[Ca2(PO3)6] + 4NaCl
2MgSO4 + Na2[Na4(PO3)6]→ Na2[Mg2(PO3)6] + 2Na2CO3
The water softened by the above process can be used boilers to remove boilers scale, in laundry and household washing purpose.
c) Inorganic ion exchanger – Permutit process: Inorganic salts like hydrated sodium aluminum silicates (Na2Al2Si2O8.xH2O) can exchange Ca2+, Mg2+, and Fe2+ ions of hard water with Na+ ions present in it. Such type of complex salts is found in minerals called zeolite. Permutit is an artificial zeolite. Permutit is prepared by fusing sodium carbonate, alumina, and silica. Chemically it is sodium aluminum orthosilicate( Na2Al2Si2O8, xH2O).
Na2Z + MgCl2→MgZ ↓+ 2NaCl
Na2Z + CaCl2→CaZ ↓+ 2NaCl
When all the permutit is exhausted, it is again converted to sodium zeolite by passing 10% NaCl solution through it.
CaZ + 2NaCl→CaCl2 + Na2Z
MgZ + 2NaCl→MgCl2 + Na2Z (where Z= Al2Si2O8.xH2O)
This method is preferred as the process is cheap, efficient and removes both temporary and permanent hardness. This method is also known as base exchange or zeolite process.
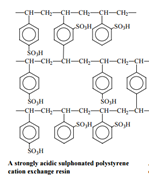
d)Organic ion exchanger: Ion exchange resins are giant organic molecules with high molecular mass, that are capable of exchanging particular ions. This method is superior to permutit process as all the types of cations(Ca2+, Mg2+ and Na+) and anions(Cl- , SO42- and HCO32-) can be removed by this process resulting deionized or demineralized water.
The organic ion exchanger used in this process is of two types:
Cation exchange resins:Cation exchangers are resins with –COOH or –SO3H group joined to the giant structure. These resins can exchange H+ with Ca2+ and Mg2+.
RCOOH + CaCl2→(RCOO)2Ca + 2H+ + 2Cl–
RCOOH + MgSO4→(RCOO)2Mg + 2H+ + SO42-
R is the giant hydrocarbon framework.
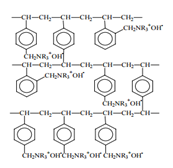
Anion exchange resins:Anion exchangers are resins with –OH group joined to the giant structure.These resins, RNH3OH can exchange Cl– and SO42-.
RNH3OH + Cl–→RNH3Cl + OH–
2RNH3OH + SO42-→(RNH3)2SO4 + 2OH–
The liberated OH– ions neutralise the H+ ions produced in the cation exchange resin. Thus, the water collected from anion exchange resin is free from all cations and anions is what called as deionised or demineralised water.
Regeneration of resins:
The resins when get exhausted are regenerated by treating the exhausted resins with concentrated sulphuric or hydrochloric acid and sodium hydroxide solution.
(RCOO)2Ca + 2HCl→2RCOOH + CaCl2
RNH3Cl +NaOH→RNH3OH + NaCl
Advantages of organic ion exchanger: The cation and anion exchangers can be used again and again. It makes this process both efficient and cheap. The demineralised water obtained by this process is as pure as distilled water and can be used instead of distilled water in different laboratories and industries.
Disadvantages of organic ion exchanger:The water produced by this process contain some dissolved gases and non-electrolytes like urea, sugar etc.
Comparison between permutit process and ion exchange resin process:
Both the process softens water by ion exchange.
In permutit process, Ca2+, Mg2+, Fe2+ are exchanged by Na+ ion , while in ion exchange resin process, Ca2+, Mg2+, Fe2+ are exchanged by H+ ion.
In permutit process, Na+ ions and anions are not removed from water, while in ion exchange resin process; both the metallic and non-metallic ions are completely removed from water.
Identification of water:
- When white anhydrous copper sulfate is treated with water, it turns blue.
- Water turns blue colored silica gel (SiO2.xH2O) containing Co(II) salts reddish-pink.
- With calcium, carbide water produces acetylene, which burns with a bright luminous light.
Degree of hardness:
Degree of hardness is defined as the number of parts by mass of calcium carbonate or equivalent to various calcium and magnesium salts present in a million parts of water by mass.It is expressed in ppm(parts per million)
| Soft water | 0-5 ppm |
| Medium hard water | 10-20ppm |
| Hard water | 20-30ppm |
| Very hard water | Above 30 ppm |
Estimation of degree of hardness:
Hard water is titrated with EDTA (Ethylenediamine tetra acetic acid)(containing buffer NH4Cl+ NH4OH solution of pH 10) with Eriochrome Black T indicator. When all the Ca2+ and Mg2+ ions are consumed, the colour of the indicator changes from wine red to blue with a drop of EDTA.

Advantages of hard water:
Due to the presence of different minerals and salts in hard water, it is tasty. It can be used to make different beverages. Ca2+ and Mg2+ ions present in hard water are essential for the growth of our body.
Lead gets dissolved in soft water as soluble lead salts when soft water is passed through lead pipes. These soluble lead salts are poisonous for us. Calcium sulphate present in hard water form insoluble lead sulphate inside lead pipe and thus prevent lead poisoning.
Disadvantages of using hard water:
Hard water is not suitable for cooking. It spoils the lustre of utensils. An incrustation is formed inside the utensils while cooking, which causes wastage of fuels.
Hard water is not suitable for washing clothes. As hard water prevent lather formation. Iron salts present in hard water causes yellow stains in clothes.
Hard water should not be used to generate steam in boilers. When hard water is boiled, CaCO3, silica, iron and aluminium oxides are precipitated. These insoluble substances are collected inside the boilers as hard scale. This layer of scale acts as heat insulator causing wastage of heat. The layer of scale and the metal of boiler expand unequally on heating. As a result, cracks are formed on the scales. Through this cracks, water reach the hot iron surface and get converted to steam. The excess pressure produced due to steam may burst the boiler.
Numericals related to the hardness of water:
1 MgSO4 ≡ 1 CaCO3 1MgCl2≡ 1 CaCO3
120ppm 100ppm 95ppm 100ppm
1CaSO4≡ 1 CaCO3 1CaCl2≡ 1 CaCO3
136ppm 100ppm 111ppm 100ppm
1 Ca(HCO3)2≡ 1 CaCO3 1 Mg(HCO3)2≡ 1 CaCO3
162 ppm 100ppm 146ppm 100ppm
One liter of a sample of hard water contains 1 mg of CaCl2 and 1 mg of MgCl2. Find the hardness of the water.
Ans: 111 g of CaCl2≡ 100 g of CaCO3
1 g of CaCl2≡ 100/111 g of CaCO3 = 0.9 g of CaCO3
1 mg of CaCl2≡ 0.9 mg of CaCO3
95 g of MgCl2≡ 100 g of CaCO3
1 g of MgCl2≡ 100/95 g of CaCO3 = 1.05 g of CaCO3
1 mg of MgCl2≡ 1.05 mg of CaCO3
Now, 1L of water contains (0.9 + 1.05) mg of CaCO3
106 mg contains 1.95mg of CaCO3
[As density of water is 1g/cc, 1L of water=106 mg]
The degree of harness of water is 1.95 ppm.
A sample of water contains 34 ppm of CaSO4 and 19 ppm of MgCl2. Calculate the total hardness of the water.
Ans: 1CaSO4≡ 1 CaCO3
136ppm 100ppm
34 ppm 25 ppm
1MgCl2≡ 1 CaCO3
95ppm 100ppm
19 ppm 20ppm
Total hardness of water= Hardness due to CaSO4 and MgCl2 = 25+20 ppm = 45ppm
One litre of a sample of water contains 12 mg Mg2+ and 20 mg Ca2+ ions as chloride salts. Determine the degree of hardness of the sample of water.
Ans: 12 mg Mg2+ = 0.012 g Mg2+ = 0.05g CaCO3[ 24g Mg2+ ≡ 95g MgCl2 ≡ 100g CaCO3]
20 mg Ca2+ = 0.02 g Ca2+ = 0.05 g CaCO3[ 40g Ca2+≡ 111g CaCl2 ≡ 100g CaCO3]
Total quantity of CaCO3 in 1L or 1000g of water = 0.05+0.05g= 0.1g
106 g water contains (0.1 x 106)/ 103 = 100 g CaCO3
The degree of harness of water is 100 ppm.
Calculate the degree of hardness of a sample of hard water containing 48mg of magnesium sulphate per kg of water.
Ans: 48 mg MgSO4 = 0.048g MgSO4
Now, 120g MgSO4 ≡ 100g CaCO3
0.048g MgSO4 ≡ 0.04g CaCO3
1kg or 1000g water contains 0.04g CaCO3
106g water contains (0.04x 106)/ 103 = 40 g CaCO3
The degree of harness of water is 40 ppm.










