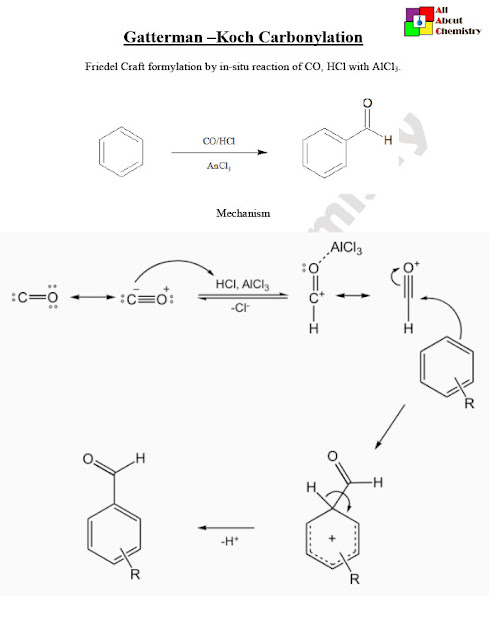
The Gattermann-Koch carbonylation is a chemical reaction used in organic synthesis to convert aromatic compounds into aldehydes or ketones using carbon monoxide (CO) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst, typically aluminum chloride (AlCl3). This reaction was developed by two different chemists, namely Gattermann and Koch, hence the combined name.
The general reaction scheme for the Gattermann-Koch carbonylation is as follows:
Ar-H+CO+HCl+AlCl3→Ar-CO-Cl→Ar-CHO or Ar-CO-R
In this reaction, Ar-H represents an aromatic compound (such as benzene or substituted benzene), which reacts with carbon monoxide (CO) and hydrogen chloride (HCl) in the presence of aluminum chloride (AlCl3) as a catalyst. Initially, the reaction forms an acyl chloride intermediate (Ar-CO-Cl), which upon further treatment, such as hydrolysis or reduction, can yield either an aldehyde (Ar-CHO) or a ketone (Ar-CO-R), depending on the reaction conditions and the nature of the aromatic compound.
The Gattermann-Koch carbonylation is particularly useful for the synthesis of aromatic aldehydes and ketones, which are important intermediates in the production of pharmaceuticals, fragrances, and fine chemicals. It provides a practical route for introducing carbonyl functional groups onto aromatic rings, enabling the synthesis of a wide range of organic compounds.
The mechanism of the Gattermann-Koch carbonylation involves several steps and intermediates. Here’s a simplified mechanism for the conversion of an aromatic compound (Ar-H) into an acyl chloride intermediate, which can subsequently yield either an aldehyde or a ketone:
- Formation of the Acyl Chloride Intermediate: Ar-H+CO+HCl+AlCl3→Ar-CO-Cl. This step involves the activation of carbon monoxide (CO) by the Lewis acid catalyst, aluminum chloride (AlCl3), to generate a highly electrophilic acyl chloride species (Ar-CO-Cl). The aromatic compound (Ar-H) undergoes electrophilic aromatic substitution with the acyl chloride, leading to the formation of an aryl acyl chloride intermediate.
- Hydrolysis or Reduction of the Acyl Chloride: The aryl acyl chloride intermediate (Ar-CO-Cl) can undergo further reaction to yield the desired product. Two common pathways are: a. Hydrolysis to yield an Aldehyde: Ar-CO-Cl+H2O→Ar-CHO+HCl . In this pathway, the aryl acyl chloride reacts with water (H2O) to yield an aldehyde (Ar-CHO) and hydrochloric acid (HCl). This step involves the nucleophilic attack of water on the acyl chloride, followed by elimination of HCl to form the aldehyde. b. Reduction to yield a Ketone: Ar-CO-Cl+R2NH→Ar-CO-NHR2+HCl. Alternatively, the aryl acyl chloride can undergo reduction, typically using a reducing agent such as a secondary amine (R2NH), to yield a ketone (Ar-CO-R) and HCl. This reduction involves the transfer of a hydride ion from the reducing agent to the carbonyl carbon, followed by protonation to form the ketone.
These steps illustrate the key transformations involved in the Gattermann-Koch carbonylation mechanism, leading to the formation of aldehydes or ketones from aromatic compounds. The choice between hydrolysis and reduction pathways depends on the desired product and specific reaction conditions.
The Gattermann-Koch carbonylation reaction finds several important applications in organic synthesis, particularly in the preparation of aldehydes and ketones from aromatic compounds. Here are some notable applications:
- Synthesis of Aromatic Aldehydes and Ketones: The primary application of the Gattermann-Koch carbonylation is in the synthesis of aromatic aldehydes and ketones. It provides a convenient and efficient method for introducing carbonyl functional groups onto aromatic rings, allowing access to a wide range of substituted aromatic aldehydes and ketones.
- Pharmaceutical Synthesis: Aldehydes and ketones synthesized via the Gattermann-Koch carbonylation reaction are valuable intermediates in the synthesis of pharmaceutical compounds. These functional groups are frequently found in drug molecules and serve as key structural motifs for imparting biological activity.
- Flavors and Fragrances: Aromatic aldehydes and ketones are essential components in the synthesis of flavors and fragrances. The Gattermann-Koch carbonylation offers a versatile approach for the preparation of these compounds, enabling the synthesis of various aroma compounds used in food, cosmetics, and perfumery.
- Fine Chemical Synthesis: The reaction is employed in the synthesis of fine chemicals and specialty compounds used in diverse industrial applications. Aromatic aldehydes and ketones serve as important building blocks for the production of specialty chemicals, agrochemicals, and materials.
- Research and Method Development: The Gattermann-Koch carbonylation continues to be an area of research interest, with ongoing efforts focused on developing improved reaction conditions, catalyst systems, and synthetic strategies. Novel applications and modifications of the reaction are explored to expand its utility and scope in organic synthesis.
- Total Synthesis of Natural Products: The reaction plays a role in the total synthesis of natural products containing aromatic aldehyde or ketone functionalities. Chemists utilize the Gattermann-Koch carbonylation as a key step in synthetic routes to access complex natural product scaffolds.
- Functional Group Interconversion: Aromatic aldehydes and ketones synthesized through the Gattermann-Koch carbonylation can undergo further functional group transformations, enabling the synthesis of diverse organic compounds with tailored properties and functionalities.
Overall, the Gattermann-Koch carbonylation reaction serves as a valuable tool in organic synthesis, offering a versatile method for the preparation of aromatic aldehydes and ketones with broad applications in various fields of chemistry and industry.








