The Baker-Venkataraman Rearrangement is a chemical reaction named after the chemists Thomas Baker and Krishnamurthy Venkataraman. It involves the rearrangement of certain arylamines (compounds containing an aryl group attached to an amino group) to form aryl hydroxylamines.
The reaction typically proceeds via a radical mechanism. In the first step, the arylamine undergoes a homolytic cleavage of the N–N bond, generating an aryl radical and a nitrogen radical. The aryl radical then migrates to the nitrogen atom, forming a nitrogen-centered radical intermediate. Finally, this intermediate undergoes capture by a suitable trapping agent, such as oxygen or a radical acceptor, to yield the desired aryl hydroxylamine.
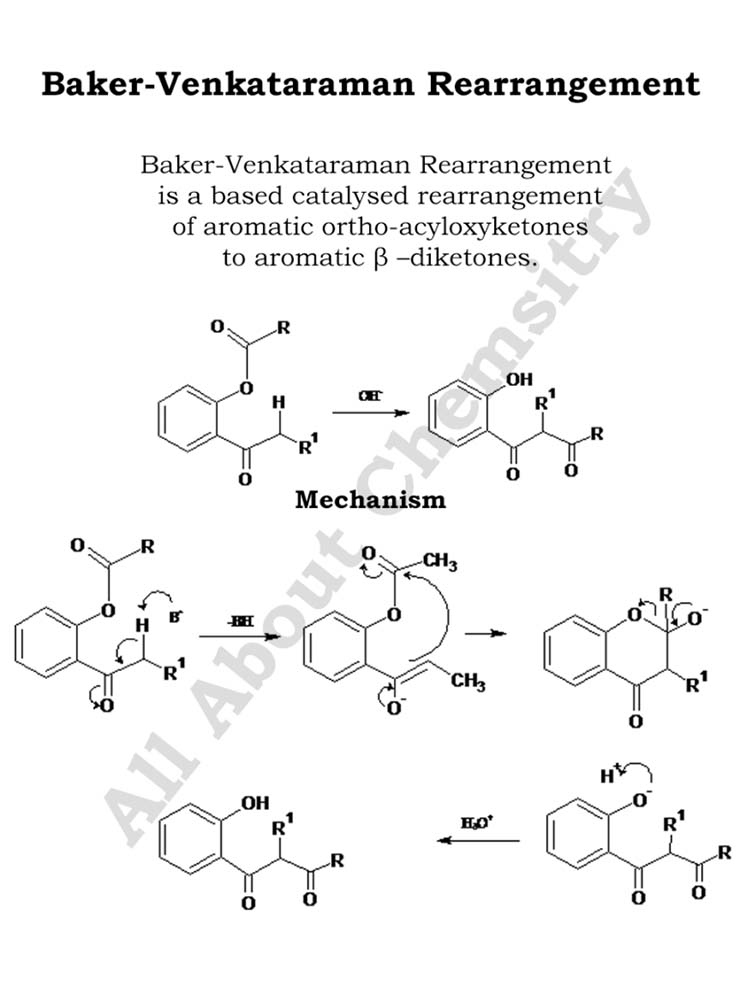
The Baker-Venkataraman Rearrangement has found several applications in organic synthesis due to its ability to introduce hydroxylamine functional groups into aromatic compounds. Some notable applications include:
- Synthesis of Nitrones: Aryl hydroxylamines formed through the Baker-Venkataraman Rearrangement can be readily converted into nitrones, which are versatile intermediates in organic synthesis. Nitrones participate in a variety of reactions including cycloadditions, 1,3-dipolar cycloaddition reactions, and various transformations leading to the formation of nitrogen-containing heterocycles.
- Preparation of Oximes: Aryl hydroxylamines obtained from the rearrangement can be converted into oximes, which are important intermediates in the synthesis of various nitrogen-containing compounds including pharmaceuticals, pesticides, and flavor compounds. Oximes can be further functionalized to introduce additional substituents or undergo rearrangement reactions leading to diverse product formation.
- Synthesis of Azo Compounds: Aryl hydroxylamines can be converted into azo compounds through diazotization and coupling reactions. Azo compounds have widespread applications as dyes, pigments, and pharmaceuticals.
- Preparation of Nitroso Compounds: Aryl hydroxylamines can be oxidized to nitroso compounds, which are valuable intermediates in the synthesis of pharmaceuticals, agrochemicals, and natural products. Nitroso compounds can undergo various transformations including cycloaddition reactions, rearrangements, and functional group interconversions.
- Synthesis of Hydroxamic Acids: Aryl hydroxylamines can be converted into hydroxamic acids, which are important chelating agents and inhibitors of various metalloenzymes. Hydroxamic acids have applications in medicinal chemistry, bioinorganic chemistry, and as industrial chemicals.
- Functionalization of Aromatic Compounds: The Baker-Venkataraman Rearrangement provides a method for the direct introduction of hydroxylamine functional groups onto aromatic rings. These hydroxylamines can serve as versatile intermediates for further functionalization, enabling the synthesis of diverse compounds with tailored properties.
The Baker-Venkataraman Rearrangement is primarily utilized in laboratory settings and industrial processes rather than in daily life. However, its applications indirectly impact daily life through the synthesis of various compounds that find use in everyday products and activities. Here are a few ways in which the Baker-Venkataraman Rearrangement contributes to daily life:
- Pharmaceuticals: The rearrangement is employed in the synthesis of intermediates that are used in the production of pharmaceuticals. Many drugs that people use daily, such as antibiotics, antiviral medications, and pain relievers, may involve compounds synthesized using methods that include the Baker-Venkataraman Rearrangement.
- Agrochemicals: The rearrangement is utilized in the synthesis of agrochemicals, including pesticides and herbicides. These chemicals play a crucial role in agriculture, contributing to the production of food and ensuring crop protection, indirectly impacting daily life through the availability of safe and abundant food supply.
- Dyes and Pigments: Azo compounds, which can be synthesized using the Baker-Venkataraman Rearrangement, are widely used as dyes and pigments in various everyday products such as textiles, paints, inks, and cosmetics. These products enhance aesthetics and functionality in daily life.
- Flavor and Fragrance Compounds: Aromatic compounds synthesized through the Baker-Venkataraman Rearrangement are used in the production of flavorings and fragrances found in foods, beverages, personal care products, and household items. These compounds contribute to the sensory experience in daily life.
- Industrial Chemicals: The rearrangement is employed in the synthesis of various industrial chemicals, including intermediates used in the production of plastics, polymers, and specialty chemicals. These materials are ubiquitous in daily life, found in packaging, construction materials, electronics, and countless other consumer products.
While the Baker-Venkataraman Rearrangement itself may not be directly evident in daily life, the compounds synthesized using this reaction have far-reaching impacts on various aspects of modern living, including healthcare, agriculture, manufacturing, and consumer goods.








