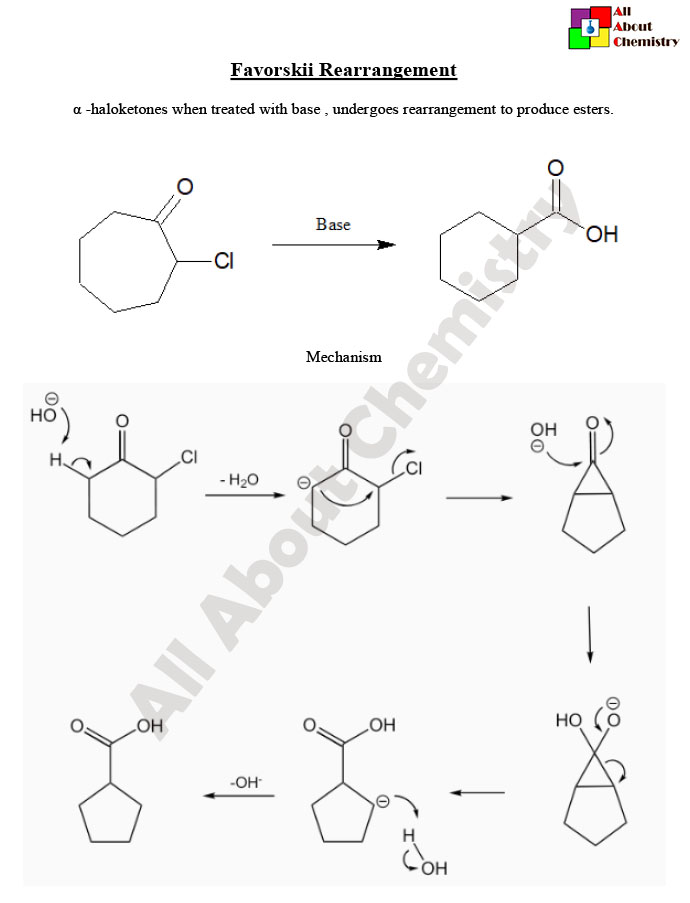
The Favorskii rearrangement is a chemical reaction named after the Russian chemist Alexei Yevgrafovich Favorskii. It involves the rearrangement of a α-halo ketone (or ester) to an α-halo carboxylic acid under basic conditions. The reaction proceeds via the migration of the halogen atom from the α-carbon to the carbonyl carbon, resulting in the formation of a carboxylic acid group.
The general mechanism of the Favorskii rearrangement involves several steps:
- Deprotonation: The α-halo ketone (or ester) is deprotonated by a strong base, such as hydroxide ion (OH^-), at the α-carbon adjacent to the carbonyl group.
- Halogen migration: The deprotonated α-carbon undergoes nucleophilic attack on the carbonyl carbon, resulting in the migration of the halogen atom to the carbonyl carbon. This leads to the formation of an enolate intermediate.
- Tautomerization: The enolate intermediate undergoes tautomerization to form a carboxylate anion.
- Protonation: The carboxylate anion is protonated by water or another acidic species to yield the final product, an α-halo carboxylic acid.
The Favorskii rearrangement is an important synthetic method for the preparation of α-halo carboxylic acids, which are valuable intermediates in organic synthesis. It is commonly used in the synthesis of complex organic molecules and natural products.
The Favorskii rearrangement has several applications in organic synthesis due to its ability to form α-halo carboxylic acids, which are versatile intermediates. Some notable applications of the Favorskii rearrangement include:
- Synthesis of α-Hydroxy Carboxylic Acids: The Favorskii rearrangement can be utilized to synthesize α-hydroxy carboxylic acids by using a suitable hydroxide as the base. After the rearrangement, the resulting carboxylic acid can be reduced or further functionalized to yield various derivatives.
- Natural Product Synthesis: The Favorskii rearrangement has been employed in the synthesis of various natural products. For example, it has been used in the synthesis of biologically active compounds like prostaglandins and other complex molecules found in nature.
- Functional Group Interconversion: The Favorskii rearrangement provides a method for interconverting functional groups in organic molecules. For instance, it can be used to convert α-halo ketones or esters into the corresponding carboxylic acids, allowing for the introduction of carboxylic acid functionality into a molecule.
- Cascade Reactions: The Favorskii rearrangement can be integrated into cascade reactions to efficiently construct complex molecular frameworks. By incorporating the rearrangement step into a sequence of reactions, multiple functional groups can be installed or modified in a single synthetic operation.
- Medicinal Chemistry: α-Halo carboxylic acids obtained via the Favorskii rearrangement can serve as key intermediates in the synthesis of pharmaceuticals and drug candidates. The ability to selectively introduce halogen and carboxylic acid functionalities enables the design and synthesis of compounds with specific biological activities.
Overall, the Favorskii rearrangement is a valuable tool in organic synthesis, offering versatility and efficiency in the preparation of diverse molecular structures with important applications in fields such as medicinal chemistry, natural product synthesis, and materials science.








