The Allen-Millar-Trippett rearrangement is a chemical reaction named after the chemists who discovered it. This rearrangement involves the conversion of certain allyl aryl ethers into propenyl aryl ethers. The general reaction scheme for the Allen-Millar-Trippett rearrangement can be represented as follows:
R−CH2−CH=CH−OAr→R−CH=CH−CH2−OAr
Here, R represents an alkyl group, and Ar represents an aryl group. The reaction results in the migration of an alkyl group from the beta position to the alpha position of the allyl group, leading to the formation of a propenyl ether.
The Allen-Millar-Trippett rearrangement is typically carried out under basic conditions, and the mechanism involves a concerted [3,3]-sigmatropic rearrangement. While the exact mechanism may vary depending on the specific substrate and reaction conditions, the general steps involve the migration of the alkyl group and rearrangement of the double bond to form the propenyl ether product.
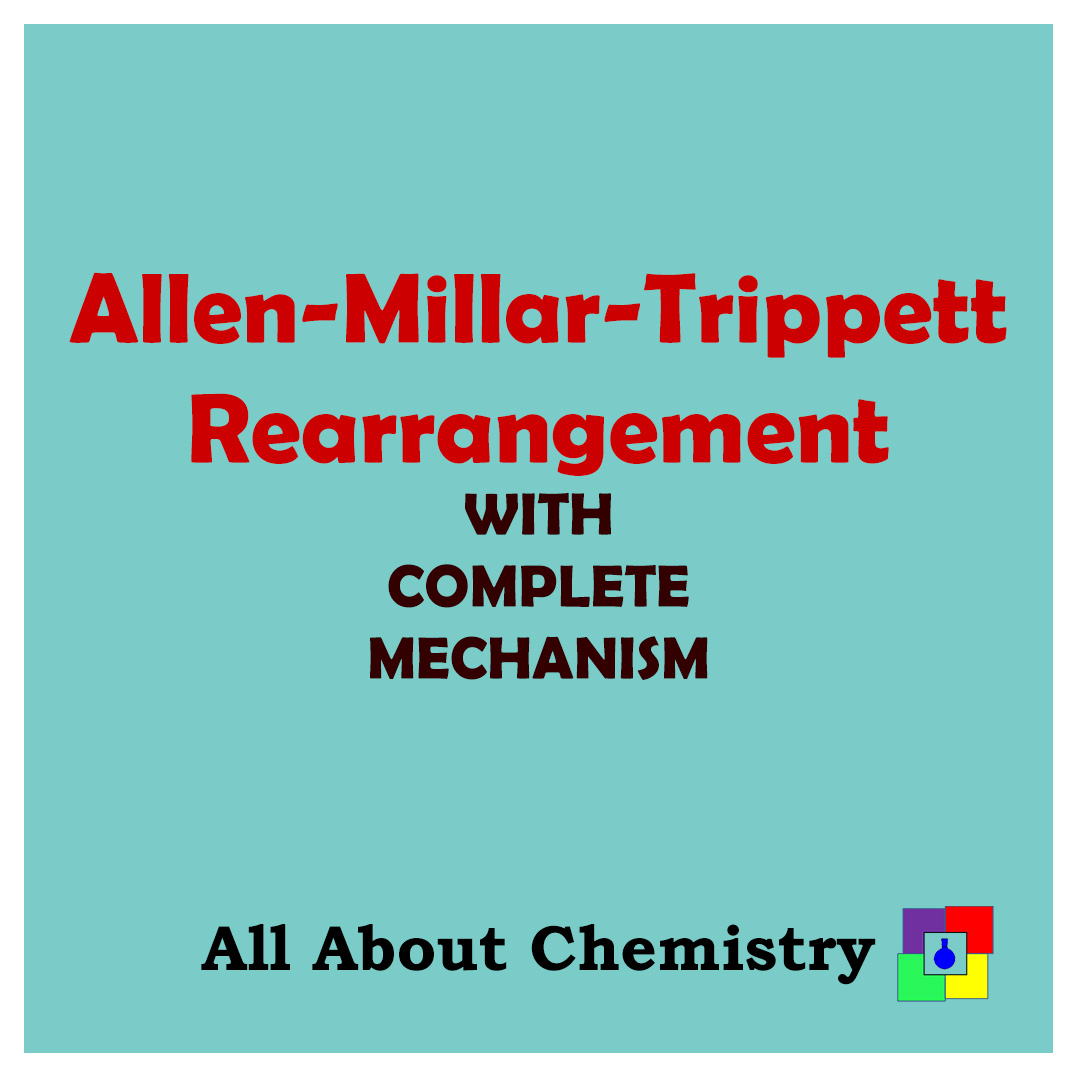
Allen-Millar-Trippett Rearrangement is the ring expansion of cyclic phosphonium salts to form dihydro phosphinine.Cyclic Phosphonium salts are obtained by reacting Cyclic phosphines with Alkyl halides or acyl halides in presence of base like triethylamine.
The Allen-Millar-Trippett rearrangement is a concerted pericyclic reaction involving a [3,3]-sigmatropic rearrangement. The mechanism proceeds through a cyclic transition state, leading to the migration of an alkyl group from the beta position to the alpha position of the allyl group, resulting in the formation of a propenyl ether. Here’s a proposed mechanism for the Allen-Millar-Trippett rearrangement:
- Proton Abstraction: The reaction begins with the deprotonation of the allyl aryl ether by a base, typically a strong base such as alkoxide ion or hydroxide ion.
R−CH2−CH=CH−OAr+B−→R−CH2−CH−=CH−OAr+BH+
- Formation of the Oxyallyl Anion: The deprotonation generates an oxyallyl anion intermediate, stabilized by resonance.
R−CH2−CH−=CH−OAr
- [3,3]-Sigmatropic Rearrangement: In a concerted manner, the alkyl group migrates from the beta carbon to the alpha carbon of the allyl system, forming a new bond between the migrating carbon and the carbon bearing the aryl group.
R−CH2−CH−=CH−OAr→R−CH=CH−CH2−OAr
- Protonation: Finally, the intermediate is protonated to yield the propenyl aryl ether product.
R−CH=CH−CH2−OAr+H+→R−CH=CH−CH2−OH+Ar−
This mechanism involves the concerted movement of electrons in the [3,3]-sigmatropic rearrangement without the formation of any discrete intermediates other than the oxyallyl anion. The Allen-Millar-Trippett rearrangement is often facilitated by the presence of electron-withdrawing groups on the aryl ring, which stabilize the oxyallyl anion intermediate. Additionally, the choice of base and reaction conditions can influence the efficiency and selectivity of the rearrangement.
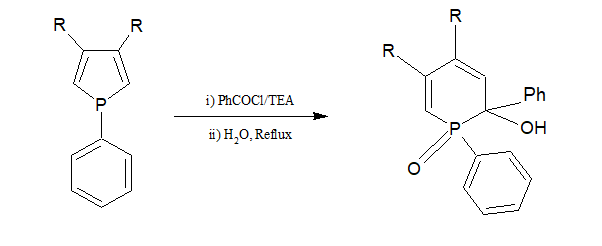
Mechanism:
The Lone electrons of the phosphorous atom of cyclic phosphine attack the carbon atom of acyl halides to form a phosphonium ion. After that, when refluxed with water, undergoes a rearrangement reaction to form dihydro phosphinine.
The Allen-Millar-Trippett rearrangement, a [3,3]-sigmatropic rearrangement, has found various applications in organic synthesis due to its ability to construct new carbon-carbon bonds and introduce functional groups into organic molecules. Some notable applications include:
- Natural Product Synthesis: The rearrangement has been employed in the synthesis of complex natural products where the formation of propenyl ethers is required. For example, it has been utilized in the synthesis of biologically active compounds like alkaloids and terpenoids.
- Functional Group Transformations: The Allen-Millar-Trippett rearrangement provides a method for the conversion of allyl aryl ethers into propenyl aryl ethers. This transformation enables the introduction of a propenyl group, which can serve as a versatile synthetic handle for further functionalization.
- Cascade Reactions: The Allen-Millar-Trippett rearrangement has been incorporated into cascade reactions, where it acts as a key step in the generation of structurally complex molecules. By coupling the rearrangement with subsequent transformations, chemists can efficiently construct diverse molecular architectures in a single synthetic sequence.
- Methodology Development: The study of the Allen-Millar-Trippett rearrangement has led to the development of new methodologies in organic synthesis. Researchers have explored different reaction conditions, substrates, and catalyst systems to expand the scope and efficiency of the rearrangement, thus enabling its application in a broader range of synthetic contexts.
- Total Synthesis of Natural Products: Due to its ability to introduce propenyl ethers, the Allen-Millar-Trippett rearrangement has been utilized in the total synthesis of various natural products. Chemists use this rearrangement as a strategic step to construct key intermediates or structural motifs present in target molecules.
- Medicinal Chemistry: The propenyl ethers generated via the Allen-Millar-Trippett rearrangement can serve as valuable intermediates in medicinal chemistry for the synthesis of pharmaceutical compounds. The ability to selectively introduce propenyl groups into molecules provides opportunities for the development of new drugs and bioactive compounds.
Overall, the Allen-Millar-Trippett rearrangement offers synthetic chemists a powerful tool for the construction of carbon-carbon bonds and the synthesis of diverse organic molecules with potential applications in drug discovery, materials science, and natural product chemistry.