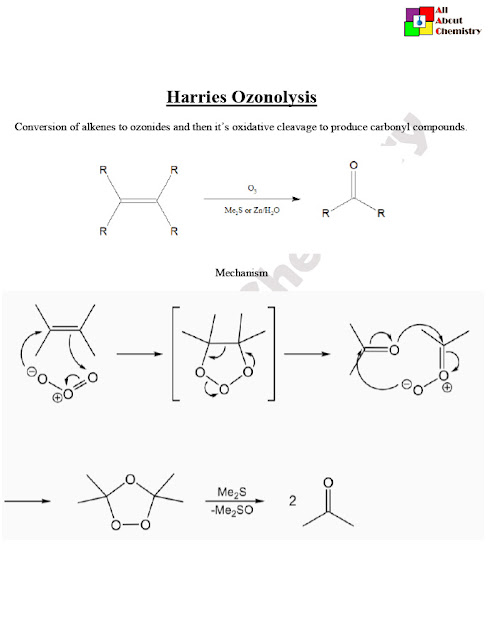
The Harries Ozonolysis, named after the German chemist Karl Harries, is a method used in organic chemistry for the oxidative cleavage of alkenes or alkynes using ozone (O3) followed by a reductive workup. This reaction is commonly employed to break carbon-carbon double or triple bonds, yielding carbonyl compounds as products.
The general reaction scheme for Harries Ozonolysis is as follows:
RCH=CHR + O3 = RCHO+RC(O)HR′
Where R and R’ represent organic substituents. The ozone (O3) is typically generated in situ by passing oxygen through a high-voltage electrical discharge, or by using a suitable ozone generator.
The Harries Ozonolysis mechanism involves several steps, leading to the cleavage of the carbon-carbon double or triple bond and the formation of carbonyl compounds. Here’s a simplified outline of the mechanism:
- Ozone Addition (1st Step):
- The alkene or alkyne reacts with ozone (O3) to form an initial ozonide intermediate.
- This step involves the electrophilic addition of ozone to the pi bond of the alkene or alkyne.
- For example, with an alkene RCH=CHR, the ozone adds across the double bond to form a cyclic ozonide.
- Ozone Decomposition (2nd Step):
- The ozonide intermediate formed in the first step undergoes decomposition.
- The decomposition can occur through either a 1,2-ozonide rearrangement or a retro-ozonolysis pathway, depending on the specific conditions and structure of the starting material.
- Reductive Workup (Final Step):
- The resulting ozonide intermediate is then treated with a reductive workup agent to cleave the O-O bond and convert the ozonide into carbonyl compounds.
- The reductive workup typically involves a reducing agent such as zinc dust, dimethyl sulfide (Me2S), or triphenylphosphine (Ph3P).
Overall, the Harries Ozonolysis mechanism provides a means to cleave carbon-carbon double or triple bonds and generate carbonyl compounds from alkenes or alkynes. The specifics of the mechanism may vary depending on factors such as reaction conditions, substituents on the starting material, and the choice of reductive workup agent.
he Harries Ozonolysis reaction finds various applications in both synthetic organic chemistry and analytical chemistry. Here are some of its key applications:
- Functional Group Transformation: Harries Ozonolysis is widely used to cleave carbon-carbon double or triple bonds, allowing for the conversion of alkenes or alkynes into carbonyl compounds. This transformation is valuable in synthetic organic chemistry for the introduction of carbonyl functionalities, which can serve as versatile intermediates for further functionalization.
- Structural Elucidation: In analytical chemistry, Harries Ozonolysis is employed for the structural elucidation of unknown compounds. By subjecting a sample containing an unknown alkene or alkyne to Harries Ozonolysis followed by analysis of the resulting carbonyl compounds, chemists can deduce the structure of the original molecule based on the known reactivity patterns of functional groups.
- Natural Product Synthesis: The ability of Harries Ozonolysis to selectively cleave carbon-carbon double or triple bonds makes it valuable in the synthesis of complex natural products. By strategically incorporating this reaction into synthetic routes, chemists can access key intermediates or fragments necessary for the assembly of target molecules.
- Polymer Chemistry: In polymer chemistry, Harries Ozonolysis can be utilized for the controlled degradation or modification of polymers containing carbon-carbon double bonds. By selectively cleaving these bonds, it is possible to tailor the properties of polymers or generate functional groups for further modification.
- Ozonolysis with Chiral Alkenes: Harries Ozonolysis can also be applied to chiral alkenes, providing a method for the asymmetric synthesis of carbonyl compounds. By using chiral starting materials or chiral reagents, it is possible to induce stereochemical control in the formation of the resulting carbonyl compounds, enabling the synthesis of enantiomerically enriched products.
Overall, the Harries Ozonolysis reaction offers a versatile tool for organic chemists, with applications ranging from fundamental organic synthesis to advanced materials science and chemical analysis.










