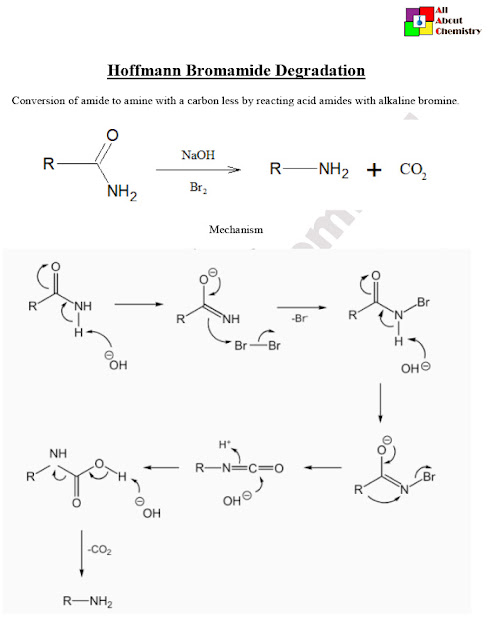
The Hoffmann bromamide degradation, also known as the Hoffmann degradation, is a chemical reaction used to convert primary amides into primary amines. This reaction is named after August Wilhelm von Hoffmann, who discovered it. The Hoffmann bromamide degradation involves treating a primary amide with bromine (Br2) and a strong base, typically sodium hydroxide (NaOH) or potassium hydroxide (KOH), in aqueous or alcoholic solution.
The general reaction scheme for the Hoffmann bromamide degradation is as follows:
RCONH2+Br2+4OH−→RNH2+CO2+2Br−+2H2O
In this reaction:
- RCONH₂ represents the primary amide.
- Br₂ is bromine.
- OH⁻ is the hydroxide ion.
- RNH₂ represents the primary amine.
- CO₂ is carbon dioxide.
- Br⁻ is bromide ion.
- H₂O is water.
The Hoffmann bromamide degradation mechanism involves several steps, starting with the bromination of the primary amide followed by alkaline hydrolysis, rearrangement, and hydrolysis of the resulting isocyanate intermediate. Here’s a detailed explanation of each step:
- Bromination: The primary amide (RCONH2RCONH2) reacts with bromine (Br2Br2) in the presence of a base (OH−OH−) to form an N-bromoamide intermediate (RCONHBrRCONHBr). The base deprotonates the amide nitrogen, making it nucleophilic and allowing it to attack the bromine molecule.RCONH2+Br2→RCONHBr+HBr
- Alkaline Hydrolysis: The N-bromoamide intermediate undergoes alkaline hydrolysis in the presence of hydroxide ions (OH−OH−). The hydroxide ion attacks the carbonyl carbon of the N-bromoamide, leading to the formation of a carbamate intermediate (RCONH2−RCONH2−).RCONHBr+OH−→RCONH2−+Br−
- Rearrangement (Hoffmann Rearrangement): The carbamate intermediate undergoes rearrangement, known as the Hoffmann rearrangement. In this step, an alkyl group migrates from the nitrogen atom to the carbonyl carbon, leading to the formation of an isocyanate intermediate.
- Hydrolysis of Isocyanate: The isocyanate intermediate undergoes hydrolysis in the presence of water (H2OH2O), yielding the primary amine (RNH2RNH2) and carbon dioxide (CO2CO2). RCONH2−+H2O→RNH2+CO2
Overall, the Hoffmann bromamide degradation mechanism involves a series of nucleophilic substitution, rearrangement, and hydrolysis steps. This reaction provides a useful method for converting primary amides into primary amines.
The Hoffmann bromamide degradation is an important reaction in organic synthesis with several practical applications:
- Amine Synthesis: The primary application of the Hoffmann bromamide degradation is the conversion of primary amides into primary amines. Primary amines are versatile building blocks in organic chemistry and are widely used in the synthesis of pharmaceuticals, agrochemicals, and various other organic compounds. The Hoffmann bromamide degradation provides a straightforward and efficient method for the synthesis of primary amines from readily available amide precursors.
- Functional Group Interconversion: The reaction can serve as a method for interconverting functional groups. For example, primary amides can be readily converted into primary amines, which can then be further transformed into other functional groups through various synthetic transformations. This flexibility makes the Hoffmann bromamide degradation a valuable tool in organic synthesis for the modification of complex molecules.
- Chemical Biology and Drug Discovery: Primary amines are common functional groups found in many biologically active compounds, including drugs and natural products. The ability to efficiently convert amides to amines using the Hoffmann bromamide degradation allows chemists to access diverse amine-containing compounds for biological evaluation and drug discovery studies. This reaction has been employed in the synthesis of various pharmaceutical intermediates and bioactive molecules.
- Peptide Synthesis: The Hoffmann bromamide degradation has also been utilized in peptide synthesis. It can serve as a method for introducing specific amino acid residues into peptide chains, particularly when primary amines are required at certain positions. By converting amide-containing peptide intermediates to their corresponding amine derivatives, chemists can achieve precise control over the sequence and structure of peptides during synthesis.
- Functionalization of Organic Compounds: Primary amines obtained via the Hoffmann bromamide degradation can be further functionalized to introduce additional functional groups. These functionalized amines can then be used as intermediates for the synthesis of a wide range of organic compounds, including heterocycles, polymers, and specialty chemicals.
Overall, the Hoffmann bromamide degradation is a versatile reaction with applications across various fields of organic chemistry, including synthesis, drug discovery, and materials science. Its simplicity and efficiency make it a valuable tool for synthetic chemists seeking to access primary amines and modify organic molecules.










