The Auwers–Inhoffen rearrangement is a chemical reaction named after German chemists Karl von Auwers and Hans Brockmann-Inhoffen. It involves the rearrangement of allylic alcohols to form ketones under acidic conditions. This rearrangement is particularly useful in organic synthesis for creating ketones from readily available allylic alcohols.
The general reaction scheme is as follows:
RCH=CHCH2OH→ R2CO
In this reaction, the allylic alcohol (RCH=CHCH2OH) is treated with an acid catalyst, typically a strong acid like sulfuric acid (H2SO4). Under the influence of the acid, the allylic alcohol undergoes rearrangement to form a ketone (R2C=O), with the double bond shifting from the allylic position to become part of the carbonyl group.
The mechanism of the Auwers–Inhoffen rearrangement involves protonation of the allylic alcohol, followed by rearrangement of the resulting carbocation intermediate to form a ketone. Here’s a simplified step-by-step mechanism:
- Protonation of Allylic Alcohol: The reaction begins with protonation of the allylic alcohol by an acid catalyst, typically sulfuric acid (H2SO4), to form a resonance-stabilized allylic carbocation intermediate.
RCH=CHCH2OH+H2SO4→RCH+=CHCH2OH2+RCH=CHCH2OH+H2SO4→RCH+=CHCH2OH2+
- Rearrangement: The allylic carbocation undergoes rearrangement via a 1,2-shift of a hydrogen atom from the neighboring carbon to the carbocation center. This rearrangement results in the formation of a more stable carbocation intermediate, which is typically a tertiary carbocation.
RCH+=CHCH2OH2+→RCH2=C(H)CH2OH2+RCH+=CHCH2OH2+→RCH2=C(H)CH2OH2+
- Deprotonation: The carbocation intermediate undergoes deprotonation to form the final ketone product. Deprotonation can occur either directly by a base present in the reaction mixture or via a proton transfer to a solvent molecule.
RCH2=C(H)CH2OH2+→R2C=O+H2ORCH2=C(H)CH2OH2+→R2C=O+H2O
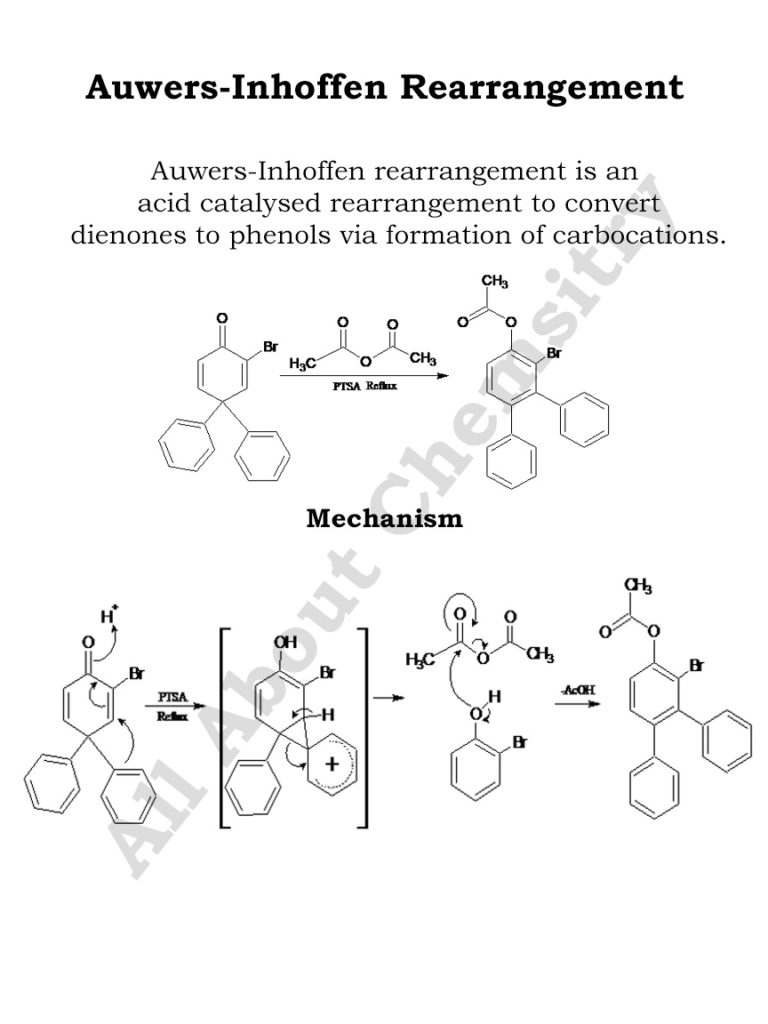
Overall, the Auwers–Inhoffen rearrangement involves the migration of a double bond and a hydrogen atom within the allylic alcohol to form a more stable carbocation intermediate, which is subsequently converted into the ketone product. The reaction proceeds through a series of acid-catalyzed steps and is favored by the stabilization of the intermediate carbocation species.
The Auwers–Inhoffen rearrangement finds application in various areas of organic synthesis. Here are a few examples:
- Natural Product Synthesis: The rearrangement is often employed in the synthesis of complex natural products. For instance, it has been utilized in the synthesis of various terpenoids, alkaloids, and other natural products where ketone functionalities are required.
- Medicinal Chemistry: In the field of medicinal chemistry, the Auwers–Inhoffen rearrangement can be used to synthesize ketone-containing compounds that serve as pharmaceutical intermediates or active pharmaceutical ingredients (APIs). These compounds may have biological activity and therapeutic potential.
- Functional Group Transformations: The rearrangement is a useful tool for converting allylic alcohols into ketones, allowing for the introduction of ketone functionalities into molecules. This can be valuable for modifying the properties of organic compounds, such as altering their reactivity or polarity.
- Total Synthesis of Natural Products: Total synthesis of complex natural products often requires strategic transformations of functional groups. The Auwers–Inhoffen rearrangement can be a key step in such syntheses, enabling the construction of key intermediates or the final target molecules.
- Heterocyclic Chemistry: The rearrangement can be employed in the synthesis of heterocyclic compounds containing ketone functionalities. This is important in the construction of diverse heterocyclic scaffolds with varied applications in materials science, pharmaceuticals, and agrochemicals.
- Methodology Development: Chemists may also explore variations or improvements to the Auwers–Inhoffen rearrangement as part of method development efforts. This could involve investigating different reaction conditions, catalysts, or substrate scope to expand the utility and efficiency of the rearrangement reaction.
Overall, the Auwers–Inhoffen rearrangement serves as a versatile synthetic tool in organic chemistry, enabling the efficient conversion of allylic alcohols to ketones and finding application in diverse areas ranging from natural product synthesis to medicinal chemistry and beyond.








