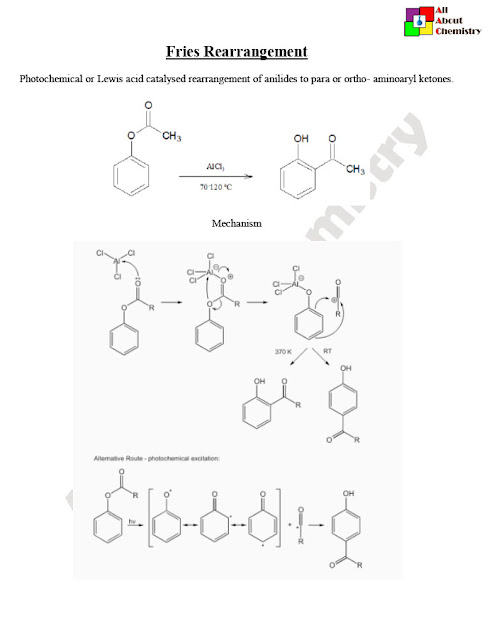
The Fries rearrangement is a chemical reaction named after the German chemist Karl Theophil Fries. It involves the migration of an acyl group (RCO–) from one position to another on an aromatic ring in the presence of a Lewis acid catalyst.
The Fries rearrangement is a rearrangement reaction involving the migration of an acyl group from one position to another on an aromatic ring. The mechanism of the Fries rearrangement typically proceeds through several steps:
- Activation of the Acyl Group: The reaction begins with the activation of the acyl group (RCO–) by a Lewis acid catalyst, often aluminum chloride (AlCl3) or another suitable Lewis acid. The Lewis acid coordinates with the carbonyl oxygen of the acyl group, making the carbonyl carbon more electrophilic and facilitating nucleophilic attack.
- Nucleophilic Attack: The activated acyl group undergoes nucleophilic attack by the aromatic ring. This attack can occur at either the ortho or para position relative to the acyl group, depending on the specific reaction conditions and steric effects. The nucleophilic attack results in the formation of a tetrahedral intermediate.
- Rearrangement: The tetrahedral intermediate undergoes a rearrangement process, which involves migration of the acyl group to a new position on the aromatic ring. This rearrangement leads to the formation of a new carbon-carbon bond between the aromatic ring and the acyl group.
- Deprotonation: The rearranged intermediate is typically protonated due to the Lewis acid catalyst present in the reaction. Deprotonation then occurs, regenerating the aromaticity of the ring and forming the final product.
Overall, the Fries rearrangement involves the activation of an acyl group, nucleophilic attack by an aromatic ring, rearrangement of the intermediate, and deprotonation to yield an ortho- or para-substituted aromatic compound containing the migrated acyl group. The reaction is an important tool in organic synthesis for the preparation of substituted aromatic compounds and finds applications in various fields, including the synthesis of pharmaceuticals, fragrances, and fine chemicals.
The Fries rearrangement finds several applications in organic synthesis, particularly in the preparation of ortho- and para-substituted aromatic compounds containing acyl groups. Here are some notable applications:
- Pharmaceutical Synthesis: The Fries rearrangement is utilized in the synthesis of pharmaceutical intermediates and active pharmaceutical ingredients (APIs). Ortho- and para-substituted aromatic compounds obtained through this rearrangement serve as key building blocks for the synthesis of various drugs. These compounds can be further elaborated to introduce additional functional groups or pharmacophores, influencing the biological activity and pharmacokinetic properties of the final drug molecules.
- Flavor and Fragrance Chemistry: The reaction is employed in the synthesis of aroma compounds used in flavors and fragrances. Ortho- and para-substituted aromatic compounds containing acyl groups contribute to the sensory attributes of food products, beverages, perfumes, and personal care items. By varying the acyl group and aromatic substituents, chemists can tailor the aroma profiles of these products to meet consumer preferences.
- Polymer Chemistry: Ortho- and para-substituted aromatic compounds obtained via the Fries rearrangement serve as monomers in the synthesis of specialty polymers. These polymers may exhibit unique properties such as solubility, thermal stability, and compatibility with other materials, making them suitable for specific applications in industries such as coatings, adhesives, and plastics.
- Fine Chemical Synthesis: The Fries rearrangement is utilized in the synthesis of fine chemicals and specialty compounds for various industrial applications. Ortho- and para-substituted aromatic compounds containing acyl groups serve as versatile intermediates for the preparation of dyes, pigments, antioxidants, lubricants, and surfactants, among other functional materials.
- Natural Product Synthesis: The reaction is employed in the total synthesis of natural products and their analogs. Ortho- and para-substituted aromatic moieties play important roles in the structures of many natural products, and their strategic introduction via the Fries rearrangement enables the construction of complex molecular architectures for biological studies and potential therapeutic applications.
Overall, the Fries rearrangement is a versatile tool in organic synthesis, offering a straightforward route to ortho- and para-substituted aromatic compounds containing acyl groups. Its applications span pharmaceuticals, flavor and fragrance chemistry, polymer science, fine chemicals, and natural product synthesis, contributing to the development of diverse molecules for various industrial and scientific purposes.








