In this section, we will study –
1.The reactions of haloalkanes
2.Preparation, and reactions of chlorobenzene
3.The physical properties of alkyl halides, and chlorobenzene.
You may also read The Preparations of alkyl halides. After completion of preparations and reactions of alkyl halides and chlorobenzene, you may also complete the PRACTICE SET.
Reactions of haloalkanes
1.Preparation of Alcohol:
Alkyl halides when reacted with Aq. NaOH or Aq. KOH or boiled with Ag2O produce Alcohol.

Depending upon the nature of the alkyl group, the reaction will follow either SN1 or SN2 mechanism.
2.Preparation of Ethers:
Alkyl halides when warmed with sodium alkoxides, ethers are formed.
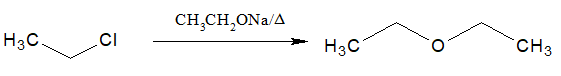
- This reaction is known as Williamson synthesis.
- 30 alkyl halides are not suitable for the preparation of ether.
- This reaction is also possible by heating with dry silver oxide.

3. Preparation of Cyanides:
Alkyl halides when heated with alcoholic KCN, alkyl cyanide is produced.
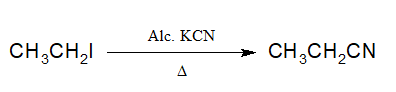
The advantage of the reaction is that CN can be hydrolysed to acid and reduced to amines.

4. Preparation of Isocyanides:
Alkyl halides when heated with alcoholic AgCN, alkyl isocyanide is produced.
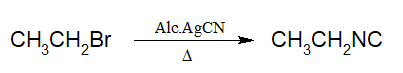
| i)Alkyl isocyanides on hydrolysis produce secondary amines. ii)CN– is an ambident nucleophile since it has two sites(carbon and nitrogen) |
5.Preparation of alkyl nitrite:
Alkyl halides when treated with NaNO2 or KNO2 produce alkyl nitrite.
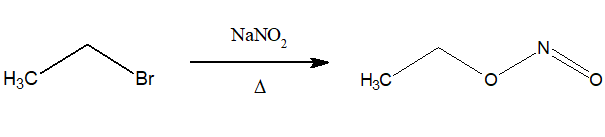
6.Preparation of nitroalkane:
Alkyl halides when treated with AgNO2 produce nitroalkanes.
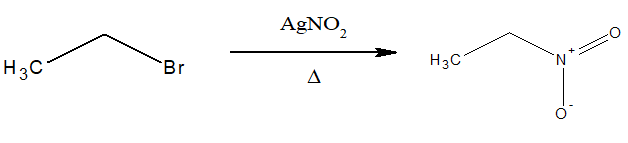
Nitrite ion is an ambident nucleophile since it has two sites (oxygen and nitrogen)
7.Preparation of amines:
Alkyl halides when treated with Ethanolic solution of ammonia at 373K, amines are produced.This reaction is called Hofmann ammonolysis.
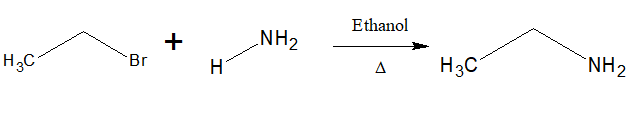
8.Preparation of higher alkynes:
Alkyl halides when treated with Sodium alkynides, higher alkynes are produced.

i)It is one of the best way to prepare alkanes of higher order.
ii)Sodium alkynides are prepared by reacting alkynes with terminal hydrogen with NaNH2/Liq NH3
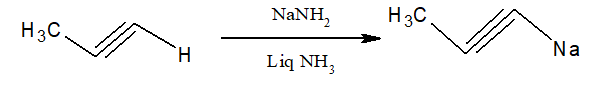
9.Preparation of alkane:
Alkyl halides when reduced, alkane is produce.
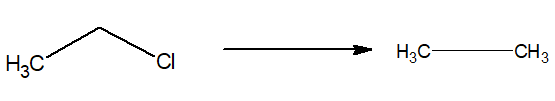
| i)LiAlH4 can reduce 10 and 20 alkyl halides. ii)NaBH4 can reduce 20 and 30 alkyl halides. iii)(Ph)3SmH can reduce all three types of halides. |
10.Preparation of alkene:
Alkyl halides when treated with alcoholic KOH, produce alkene.

| i)RCl<RBr<RI is the reactivity rate for a definite alkyl group. ii)30>20>10 iii)This rule is known as Saytzeff’s Rule. |
11.Preparation of higher alkanes:
Alkyl halides when reacted with sodium in dry etherial medium, higher alkane is produced.
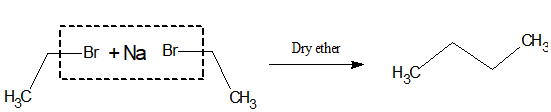
| i)This reaction is known as the Wurtz reaction. ii)Bromides and Iodides are used for it. iii)It can produce alkanes with even no carbon. iv)Tertiary halides do not respond to this test. v)One of the methods to increase the carbon chain. |
12.Preparation of Grignard Reagent:
Alkyl halides when reacted with Mg in dry ether, grignard reagent is produced.
CH3Cl + Mg → CH3MgCl
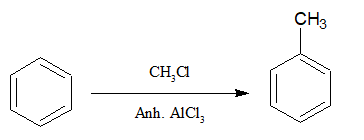
13.Preparation of aromatic substituted alkyl group:
Benzene or any aromatic compound[excluding special cases] when reacted with alkyl halides in presence of anhydrous AlCl3 , alkyl arenes or aryl alkane is produced.
14.Preparation of TEL:
Alkyl halides when reacted with Pb-Na alloy, TEL is produced.TEL or tetra ethyl lead is an antiknocking agent in petrol engine.
4CH3CH2Br + 4Na-Pb → (CH3CH2)4Pb + 4NaBr + 3Pb
15.Preparation of higher alkane:
Alkyl halides when reacted with Lithium dialkylcuprate, higher alkane is produced.
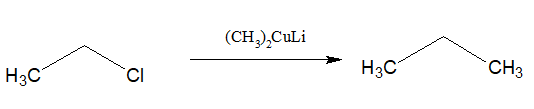
i)This reaction is called Corey House reaction.
ii)It can produce alkanes of any no of carbon atoms.
16.Haloform Reaction:
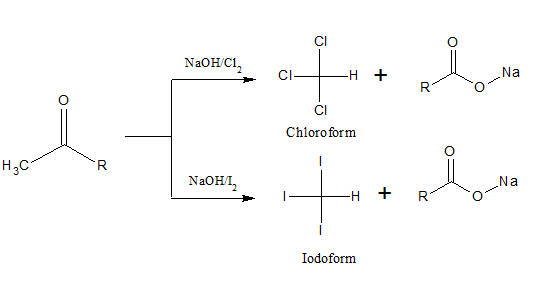
| i)Compounds containing keto methyl group when reacted with halogen in strong alkali form haloform and sodium salt of carboxylic acid with one carbon less. ii)Chloroform is a sweet-smelling colorless liquid. iii)Iodoform is a yellow solid with ‘hospital like’ smell. iv)Iodoform on heating with AgNO3 gives a yellow ppt of AgI whereas chloroform does not give a white ppt of AgCl. v)In presence of light and air, chloroform gets oxidized to poisonous phosgene(carbonyl chloride). In order to prevent it, it is kept in dark-colored bottles, with 1% ethanol. Ethanol acts as a negative catalyst. It decreases the oxidation of chloroform and also reacts with chloroform to produce non-toxic diethyl carbonate. 2CH3CH2OH + COCl2 → (CH3CH2)2CO3 + 2HCl |
Physical properties of haloalkanes
- Alkyl halides are polar covalent in nature and does not react with AgNO3 to produce a curdy white ppt. of AgCl.
- Alkyl halides are insoluble in water but soluble in organic solvent. This is due to the fact that they cannot form hydrogen bonds with water molecules and are unable to break the hydrogen bonds present among the water molecules.
- Fluoride<Chloride<Bromide<Iodide. Increasing order of density.
- RF<RCl<RBr<RI. Increasing order of boiling point.
- Tert-butyl chloride<isobutyl chloride<n-butyl chloride. More branching→more spherical shape→les surface are→vander Waal forces decreases→boiling point decreases.
- RI<RBr<RCl<RF. Stability order. Alkyl iodides break up to iodine to produce violet vapour of iodine.
- CH3Cl>CH3F>CH3Br>CH3I. Order of dipole moment.
- CH3Cl>CH2Cl2>CHCl3>CCl4. Order of dipole moment.
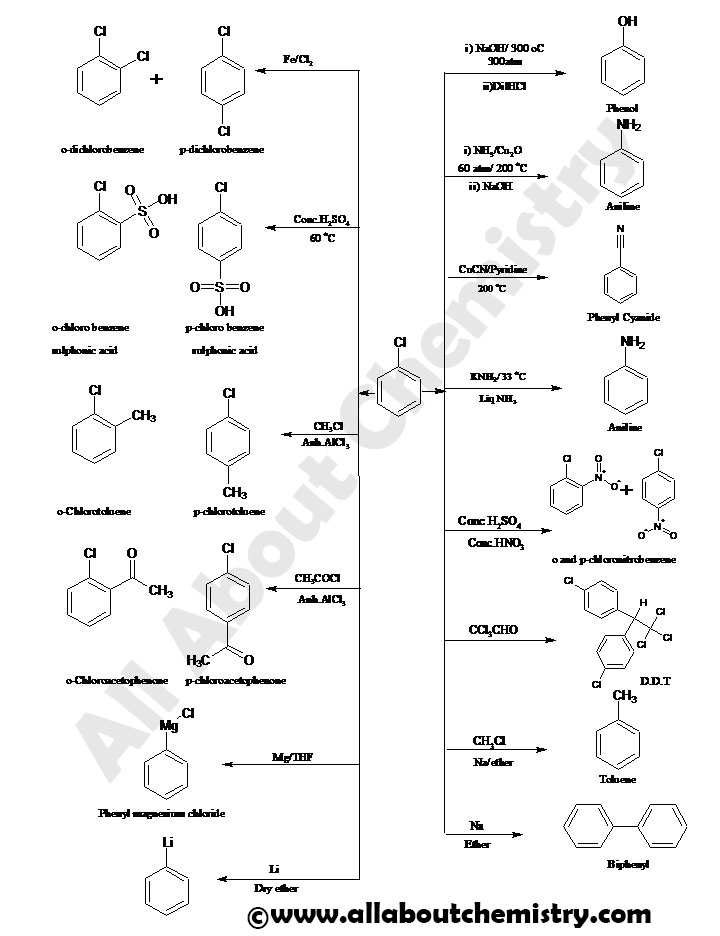
PREPARATION OF CHLOROBENZENE

REACTIONS OF CHLOROBENZENE
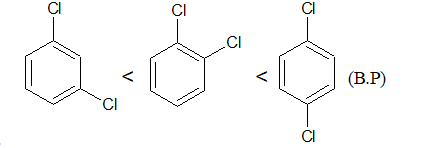
Physical properties of arylhalides
1.ArI>ArBr>ArCl>ArF>ArH ( Density , reactivity, M.P and B.P)
2.Due to resonance, C-O bond in phenol is much stronger than C-O bond in alcohol and hence, cannot be easily broken. Thus, aryl halides cannot be prepared by the action of HX, PCl3, PCl5 or SOCl2 on phenol.
3.Haloarenes are less reactive than haloalkanes because-
a) Lone pairs of chlorine gets delocalized in the benzene ring making C-Cl bond shorter.
b) The C-Cl bond has double bond character which shortens the length.
c) C in C-Cl in aryl halides is in sp2 state thus the carbon atom in C-Cl in aryl halide holds the shared electron pair more firmly.
d) Less dipole moment of aryl halides.










