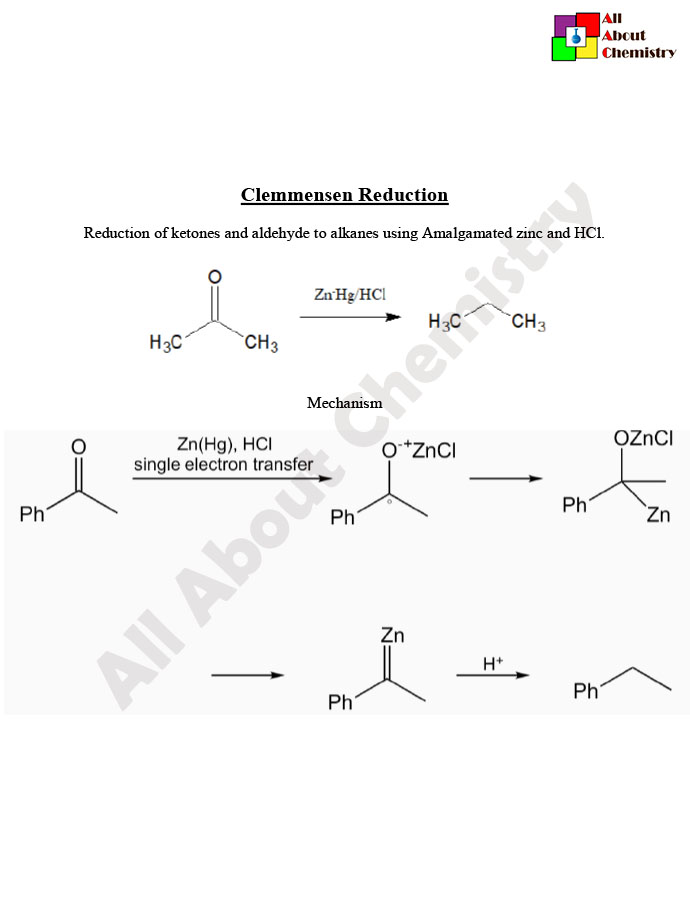
The Clemmensen reduction is a chemical reaction used to convert ketones and aldehydes into their corresponding alkanes. This reduction involves the use of zinc amalgam (zinc metal dissolved in mercury) and hydrochloric acid (HCl) as the reducing agent. The reaction is typically carried out under acidic conditions and at elevated temperatures.
The general reaction mechanism involves the formation of an organozinc intermediate, followed by protonation and subsequent elimination of the carbonyl group to yield the alkane product.
The Clemmensen reduction is particularly useful for reducing carbonyl groups that are sensitive to other reducing agents, such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4), which are more commonly used for the reduction of carbonyl groups in various organic synthesis reactions.
One limitation of the Clemmensen reduction is that it is not suitable for substrates containing sensitive functional groups that may be affected by the acidic conditions or the presence of zinc metal. In such cases, milder reducing agents or alternative reduction methods may be preferred.
Overall, the Clemmensen reduction is a valuable tool in organic synthesis for the conversion of carbonyl groups to alkanes, providing a complementary method to other reduction reactions.
The Clemmensen reduction mechanism involves the conversion of a carbonyl group (aldehyde or ketone) to an alkane using zinc amalgam (zinc metal dissolved in mercury) and hydrochloric acid (HCl) as the reducing agent under acidic conditions. Here’s a simplified stepwise mechanism:
- Formation of Organometallic Intermediate: In the presence of acid, zinc amalgam reacts with HCl to form zinc chloride and nascent hydrogen: Zn+2HCl→ZnCl2+H2 The nascent hydrogen generated reacts with the carbonyl group, leading to the formation of an organometallic intermediate. In the case of a ketone or aldehyde: R−C(=O)−R′+2H→R−CH2−R′+H2O This results in the insertion of a hydrogen atom into the carbonyl carbon, forming a carbon-zinc bond.
- Protonation and Elimination: The resulting organozinc intermediate is protonated by the acidic medium: R−CH2−R′+H+→R−CH3+R′−H This protonation step leads to the elimination of the carbonyl oxygen as water, regenerating the carbonyl-free alkane.
The overall reaction can be represented as: R−C(=O)−R′+2H→R−CH3+R′−H
It’s important to note that the reaction occurs under acidic conditions to facilitate the protonation step and to maintain the stability of the zinc amalgam. Additionally, the presence of water can hydrolyze the zinc amalgam, providing the necessary hydrogen atoms for the reduction.
Overall, the Clemmensen reduction provides a straightforward method for converting carbonyl groups to alkanes under mild conditions.
The Clemmensen reduction, a method used to convert ketones and aldehydes into corresponding alkanes, finds several applications in organic synthesis and industrial chemistry. Some of its notable applications include:
- Stereochemistry Control: Clemmensen reduction offers a means to reduce ketones or aldehydes while preserving stereochemistry, making it useful in the synthesis of chiral compounds.
- Protecting Group Strategy: It can be used as a protecting group strategy for carbonyl functionalities, allowing selective reduction of carbonyl groups in the presence of other functional groups.
- Natural Product Synthesis: Many natural products contain carbonyl functionalities that need to be selectively reduced during their synthesis. Clemmensen reduction provides a versatile method for this purpose.
- Synthesis of Hydrocarbons: In industrial applications, Clemmensen reduction can be employed for the synthesis of hydrocarbons used as solvents, fuel additives, or intermediates in the production of various chemicals.
- Reduction of Aromatic Ketones: While other reducing agents like lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4) are commonly used for reducing carbonyl groups, the Clemmensen reduction can be advantageous for reducing aromatic ketones, which are often inert to these other reducing agents.
- Heterocyclic Chemistry: Clemmensen reduction can be used in the synthesis of heterocyclic compounds where the carbonyl group is reduced to introduce a methylene group, thereby diversifying the structure of the heterocyclic ring system.
- Total Synthesis of Complex Molecules: In the total synthesis of complex molecules such as natural products or pharmaceuticals, Clemmensen reduction can play a crucial role in the construction of key intermediates or in the late-stage modification of functional groups.
Despite its usefulness, Clemmensen reduction has limitations, including its incompatibility with sensitive functional groups and the use of hazardous reagents like mercury. As a result, alternative methods such as Wolff-Kishner reduction or catalytic hydrogenation are often employed when these limitations are a concern.








