Properties of hydrogen
Physical properties of hydrogen:
| Colour | Colourless |
| Taste | Tasteless |
| Odour | Odourless |
| Density | 0.08988g/L (00C , 1atm) |
| Relative vapour pressure | 0.07 |
| Melting point | -259.350C |
| Boiling point | -252.880C |
| Solubility in water | Slightly soluble |
| Bond dissociation energy | 436 kJ/mol |
| First I.P | 1311 kJ.mol -1 |
| Speed of sound(400K, 1atm) | 1518m/s |
Chemical properties of hydrogen:
The chemical properties of hydrogen depend mainly on its bond dissociation energy. Due to high bond dissociation energy (436 kJ/mol), high energy is required to break the H-H bond. This makes hydrogen quite stable and unreactive at ordinary temperature. Majority of the reactions of hydrogen takes place at a high temperature or in presence of ultraviolet radiation.
Combustion:
Hydrogen is a combustible gas but is a non-supporter of combustion. When burnt, it burns with a pale blue flame in air or oxygen forming water. This reaction is highly exothermic (283kJ/mol)
2H2 + O2 → 2H2O
Reaction with metals:
At high temperatures, hydrogen reacts with strong electropositive metals like Na, K, Ca, etc. to produce metal hydrides.
H2 + 2Na →2NaH
H2 + Ca→CaH2
Reaction with non-metals:
Reaction with halogens:
The reactivity of hydrogen with halogens decreases with the decrease in the reactivity of the halogens. F2>Cl2>Br2>I2
Hydrogen reacts with fluorine in dark at ordinary temperature to produce hydrogen fluoride.H2 + F2 → 2HF
In the presence of sunlight, hydrogen reacts with chlorine to produce hydrogen chloride. H2 + Cl2 → 2HCl
At about 4000C, hydrogen reacts with bromine to produce hydrogen bromide. H2 + Br2 → 2HBr
At about 4000C, in presence of Pt catalyst, hydrogen reacts with iodine to produce hydrogen iodide.H2 + I2 → 2HI
- Reaction with nitrogen:
At 673K, at 200 atm pressure, in presence of Fe catalyst and Mo promoter, nitrogen reacts with hydrogen (1:3) to produce ammonia. This reaction is used to manufacture ammonia industrially by Haber’s process. N2 + 3H2 → 2NH3
- Reaction with sulphur:
At 700K, when hydrogen gas is bubbled through molten sulfur, hydrogen sulfide gas is produced.H2 + S → H2S
- Reaction with carbon:
Hydrogen reacts with carbon at 1275K to produce methane in the presence of Co or Ni catalyst. 2H2 + C→CH4
At a further higher temperature (3300K), acetylene gas is produced. 2C + H2 →C2H2
- Reduction of metal oxides and ions:
Hydrogen acts as a reducing agent. It can reduce oxides of some metals like Pb, Fe, Cu, Zn etc. to the corresponding metals.
CuO + H2 → Cu + H2O
ZnO + H2 → Zn + H2O
PbO + H2 → Pb + H2O
Fe3O4 + 4H2 → 3Fe + 4H2O
Oxides of strong electropositive elements like alkali metals and alkaline earth metals cannot be reduced by hydrogen.
Hydrogen can also reduce some metal ions (metals lying below hydrogen in the activity series) in aqueous solution.
Pd2+ + H2 → Pd + 2H+
Cu2+ + H2 → Cu + 2H+
- Reaction with carbon monoxide:

- Reaction with organic compounds:
- Unsaturated compounds such as alkenes and alkynes undergo an addition reaction with hydrogen in the presence of Pt or Pd or Ni catalyst to produce saturated compounds.
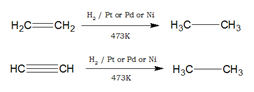
- Alkenes react with CO and hydrogen in presence of octacarbonyldicobalt catalyst under high temperature and pressure to form aldehydes. This reaction is called hydroformylation or oxo-process.
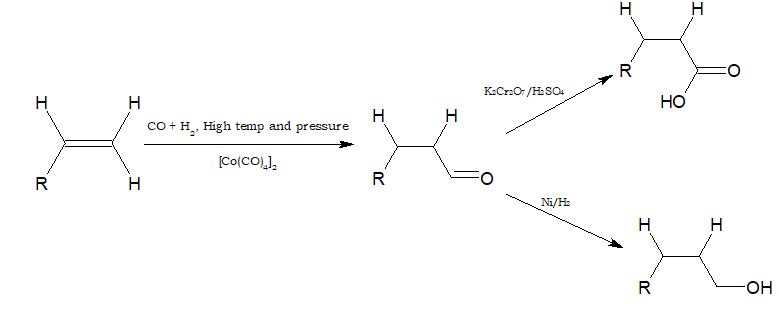
The aldehyde obtained can be oxidised to carboxylic acid and reduced to alcohol.
- Vegetable oils such as soyabean oil, groundnut oil, etc. contain double bonds in it and are called polyunsaturated oils. When these oils are exposed to air for a long time, the double bonds get oxidized and the oils become rancid. To avoid this, double bonds are hydrogenated at 473K in presence of finely divided Ni catalysts.
Vegetable oil(l) + H2 → Vegetable fat(s)
This process is called hydrogenation or hardening of oils and is used in the manufacture of vegetable ghee like Dalda, Rathetc.
Uses:
- Hydrogen is used in the manufacture of ammonia by Haber’s process.
- In the manufacture of metal hydrides, HCl, methanol and vegetable ghee(hydrogenation of vegetable oil to solid form).
- In airships and weather balloons as a mixture of hydrogen and helium (15% H2 + 85% He) is taken.
- In the manufacture of synthetic petrol by heating coal and heavy oils in the presence of the catalyst under high pressure.
- In atomic hydrogen( 4000K) and oxy-hydrogen torches(2770K) for welding and cutting of metals.
- Liquid hydrogen is used as rocket fuel in the space program.
- In metallurgy, it is used as a reducing agent to obtain certain metals like Pb, Fe, Cu, Zn etc.from the respective metal oxide.
Hydrogen as a fuel: Hydrogen Economy
The global energy consumption is expected to increase in the next decades due to the rising standards of living and a growing population worldwide. The increased need for more energy will require enormous growth in energy generation capacity, more secure and diversified energy sources, and a successful strategy to control greenhouse gas emissions. Fossil fuels such as coal, petroleum, natural gas etc. are the main source of energy in our present day. It constitute of about 80.6% of the total world energy consumption. But all these sources are limited. Thus new sources of energy are to be found out. Hydrogen can be considered as an alternate source of energy. The proposal of using hydrogen as an alternate source of energy is referred to hydrogen economy. The term was first coined by John Bockris.
Advantages of hydrogen as a fuel:
1. Hydrogen is the most abundant element in theuniverse; hence it can be easily obtained.
2. Hydrogen provides pollution free atmosphere as its combustion produce only water as the product.
3. Hydrogen is non-toxic.
4. It is more powerful and efficient than the commonly used fuels. It produces more amount of energy than other fuels.
5. It is a renewable source of energy.
Disadvantages of hydrogen as a fuel:
1. Isolating pure hydrogen in a massive scale is quite expensive.
2. Storage and transport of hydrogen is problematic.
3. It is quite expensive than the commonly used fuels.
4. Hydrogen is highly inflammable .Thus to use hydrogen as a fuel is quite risky.
5. In order to separate hydrogen from oxygen, non-renewable sources like coal, oil and natural gas are required.
Occurrence and Isotopes of Hydrogen (CLICK)
Preparation of Hydrogen (CLICK)










