Test for Unsaturation
1. Baeyer’s Test :
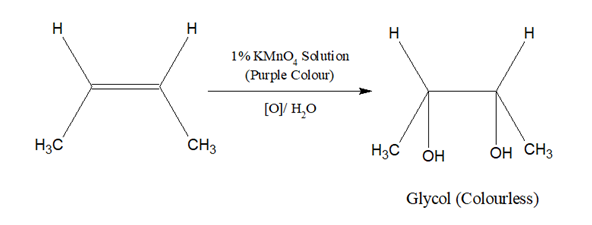
About 0.2 mL of the sample is dissolved in water or acetone to which 1% alkaline KMnO4 solution (Baeyer’s reagent ) is added.
The purple colour of KMnO4 disappears. Double bond may be present.
2.Bromine Test:
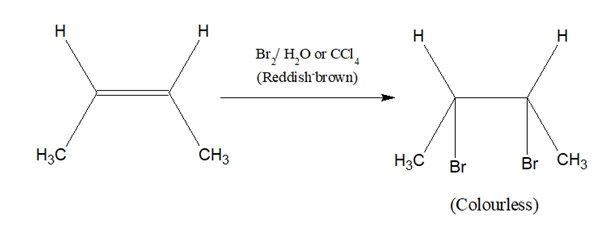
Br2/H2O or Br2/CCl4 solution is added to a test tube containing a solution or suspension of a small amount of the sample in CCl4 or in water. A glass rod dipped in NH4OH is held at the mouth of the test tube.
The reddish-brown colour of Br2 disappears, but white fumes are not formed around the glass rod.
Double or triple bond confirmed.
Test for alcoholic –OH group
1.Cerium ammonium nitrate test:
2 mL of ceric ammonium nitrate (hexanitro ammonium cerate) is added to 1-2 drops of the liquid sample or an aqueous solution of the sample.
The yellow reagent turns red.
Aliphatic –OH group may be present.
(NH4)2[Ce(NO3)6] (Yellow)+ 2R-OH → [Ce(NO3)4(ROH)2] (Red)+ 2NH4NO3
2.Xanthate Test:
Few beads of KOH is added to the sample and dissolved by heating. After cooling, 1 mL of ether and 2-3 drops of CS2 are added.
A yellow ppt is formed(Due to xanthate)
Alcoholic –OH group may be present.
ROH + KOH → ROK + H2O
ROK + CS2 → S=C(OR)(SK)(Yellow)
3.Acetyl chloride Test:
2mL sample is shaken with CaO or anhydrous MgSO4 followed by 4-5 drops of acetyl chloride. A glass rod dipped in ammonium hydroxide is held in the issuing gas.
A pungent smelling gas is liberated producing white fumes of ammonium chloride.
Alcoholic –OH group may be present.
ROH + CH3COCl → CH3COOR + HCl
HCl + NH4OH → NH4Cl (White fumes)+ H2O
4.Test with sodium metal:
2mL of the sample solution is dried by adding anhydrous MgSO4. The dried sample is decanted into another test tube. A piece of Na metal is added to this dry sample.
Effervescence of hydrogen gas is evolved.
Alcoholic –OH group may be present.
2ROH + 2Na → 2RONa + H2
5.Lucas Test:
Alcohol is reacted with a mixture of conc HCl and anh. ZnCl2.
- 3o alcohol- very rapidly turbidity is produced.
- 2o alcohol- Turbidity is produced within about five minutes.
- 1o alcohol- At room temperature, no reaction occur.
6.Victor Meyer Test:
Alcohol is treated with conc HI and red P followed by AgNO2 and then, NaNO2 + HCl . Finally NaOH is added.
- Primary alcohol- Blood Red Colour
- Secondary alcohol- Blue colour
- Tertiary alcohol- Colourless

Test for phenolic –OH group
1.Ferric Chloride Test:
To 0.2 mL of the sample, 1-2 drops of neutral FeCl3 solution is added.
The solution turns viotet or blue or green.
Phenolic –OH group may be present.
6 ArOH + FeCl3 → 3H+ [Fe(OAr)6]3- + 3HCl
2.Liebermann’s Test:
Few crystals of NaNO2 are added to about 0.5 g of the dry sample. The mixture is heated for a few minutes and cooled. The, 1 mL of conc sulphuric acid is added.
Colur of the mixture turns deep green. When this mixture is poured into excess of water, it turns red. The red solution turns blue on adding of KOH solution. Phenolic –OH group is present.

3.Back Dye Test:
1-2 drops of aniline is dissolved in dilute HCl. The solution is cooled and and diazotised with cold NaNO2 solution. Few drops of this diazotised solution is added to the alkaline solution of the sample.
Red Coloured azo dye is formed.
Presence of phenolic –OH group is confirmed.
Test for aldehyde and ketone group
1.2,4-DNPTest:
2-3 mL of 2,4-DNP (Brady’s Reagent) is added to 2mL of alcoholic solution of the sample and shaken.
Red or yellow ppt formed
Aldehyde or ketone group may be present.

2.Tollen’s Test:
About 3mL of tollen’s reagent is warmed in a hot water bath with the sample.
A shining mirror is formed on the inner surface of the test tube.
Aldehyde group is confirmed.
RCHO + 2[Ag(NH3)2]+ OH– → 2Ag (Grey or silvery mirror) + RCOO–NH4+ + H2O + 3NH3
3.Fehling Test:
Equal volume of Fehling A and B solution are mixed to produce a Fehling Solution. In it, 0.2mL of the sample is added and warmed for 4-5 mins in a hot water bath.
Brick Red ppt formed.
Aliphatic aldehyde group is confirmed.
RCHO + 2CuO → RCOOH + Cu2O(Red Ppt)
4.Benedict’s Test:
2 mL of the sample solution is warmed with Benedict’s solution for 4-5 mins in a hot water bath.
Brick Red ppt formed.
Aliphatic aldehyde group is confirmed.
RCHO + 2CuO → RCOOH + Cu2O(Red Ppt)
5.Schiff’s Test:
2-3 drops of the sample solution is added to 1mL of the reagent.
Formation of red Colour.
Alidehyde group is confirmed.
6.Iodoform test:
Few drops of iodine solution is added to the sample and then warmed in hot water bath with few drops of NaOH solution.
Yellow ppt formed.
Presence of keto methyl group is confirmed.
RCOCH3 + 3I2 + 4NaOH → 3NaI + CHI3 (Yellow) + RCOONa + 3H2O
7.Nitroprusside Test:
The the alkaline sample solution few drops od sodium nitroprusside solution is added.
Formationod a wine red colour.
Ketone group is present.
Test for carboxylic acid group
1.Sodium bicarbonate Test:
5 to 10 drops of the sample solution is added to 5mL of aqueous sodium bicarbonate solution.
Brisk effervescence of a colourless gas occurred which turns lime water milky but has no effect on acidified potassium dichromate solution.
-COOH group may be present.
RCOOH + NaHCO3 → RCOONa + H2O + CO2(effervescence)
2.Esterification Test:
mL of ethanol is added to 0.5 mL of the sample followed by 1mL of concentrated sulphuric acid and warmed in a hot water bath. The mixture is then poured into a 10mL sodium carbonate solution taken in a beaker.
Fruity smell is obtained.
Presence of –COOH group is confirmed.
CH3COOH + CH3CH2OH → CH3COOCH2CH3 (Fruity smell )+ H2O
3.Iodate-Iodide Test(Weak carboxylic acids):
Small quantity of the sample is mixed with 5% KI solution and two drops of 5% KIO3 solution. Then the mixture is warmed in a hot water bath for 2-3 mins. The content is cooled and few drops of freshly prepared starch solution is added.
A blue colour is formed.
Carboxylic acid group is present.
6RCOOH + 5KI + KIO3 → 6RCOOK + 3H2O + 3I2(Blue)
Test for amino group
1.Carbylamine Test:
2-3 drps od the sample is taken in a test tube. To it, 3-4 drops of chloroform is added followed by 2 mL of alcoholic KOH solution. The mixture is warmed.
Offensive odour is obtained.
Primary amine group is confirmed.
RNH2 + CHCl3 + 3KOH → RNC(Offensive smell) + 3KCl + 3H2O
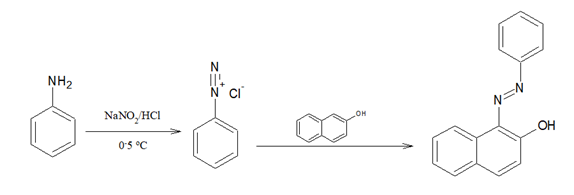
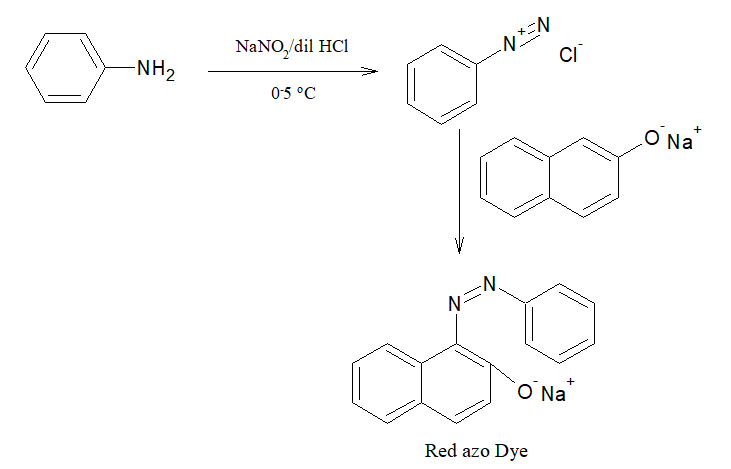
2.Dye Test:
0.3mL of the smaple is dissolved in 3mL of dilute HCl. To it, sodium nitrite solution is added. The mixture is cooled to 0-5 oC . Then alkaline β-Naphthol solution is added.
Red coloured dye is formed.
Presence of primary aromatic amine is confirmed.