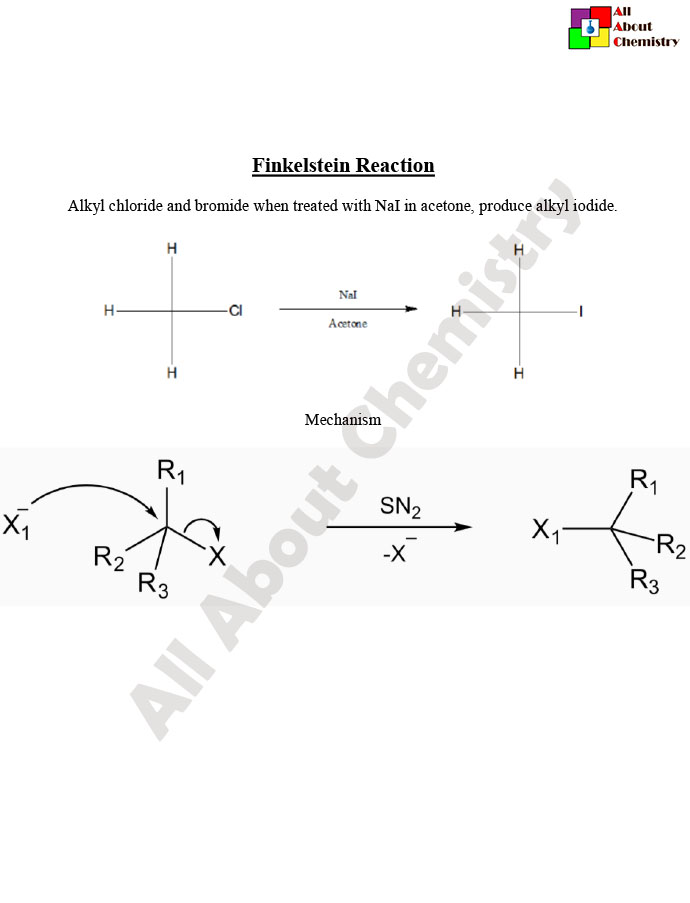
The Finkelstein reaction is a classic organic chemical transformation named after the German chemist Hans Finkelstein. It involves the conversion of an alkyl halide to another alkyl halide with a different halogen. Typically, the reaction involves the substitution of one halogen atom (such as chlorine or bromine) with another (usually iodine) in the presence of a sodium or potassium halide salt. The general reaction can be represented as follows:
R-X+NaI (or KI)→R-I+NaX (or KX)
Where R is an alkyl group and X represents a halogen atom.
The reaction is usually carried out in a polar solvent such as acetone or dimethyl sulfoxide (DMSO). The mechanism typically involves an SN2 (nucleophilic substitution) pathway, where the nucleophilic halide ion (from NaI or KI) attacks the electrophilic carbon center of the alkyl halide, displacing the leaving group to form the new alkyl iodide product.
The Finkelstein reaction is particularly useful for the synthesis of alkyl iodides, which are more reactive than their chloro- or bromo- counterparts and can undergo further transformations such as nucleophilic substitution, cross-coupling reactions, or Grignard reactions. Additionally, it is also valuable for the purification of alkyl halides, as the less reactive iodide can often be easily separated from the more reactive chlorides or bromides.
The Finkelstein reaction typically follows an SN2 (nucleophilic substitution bimolecular) mechanism. Here’s a step-by-step description of the mechanism:
- Formation of the Transition State: In the presence of a polar solvent such as acetone or DMSO, the alkyl halide (R-X) and sodium iodide (NaI) dissolve and dissociate into ions. The polar solvent helps in the solvation of ions, facilitating the reaction. The nucleophilic iodide ion (I^-) approaches the alkyl halide, leading to the formation of a transition state in which the iodide ion starts to form a bond with the carbon atom bearing the leaving group (X).
- Nucleophilic Attack: The lone pair of electrons on the iodide ion attacks the carbon atom, resulting in the formation of a new bond between carbon and iodine. Simultaneously, the leaving group (X^-) starts to depart, breaking the carbon-halogen bond.
- Formation of the Alkyl Iodide Product: As the iodide ion completes its attack on the carbon atom, the leaving group fully dissociates from the molecule, resulting in the formation of the desired alkyl iodide product (R-I).
- Solvent Interaction and Ion Pair Dissociation: The solvent molecules interact with the resulting ions (Na^+ and X^-) to stabilize them. In some cases, the solvent may also assist in the dissociation of the ion pair, facilitating the reaction.
Overall, the Finkelstein reaction mechanism proceeds via a concerted, single-step process in which the nucleophilic iodide ion directly displaces the leaving group from the alkyl halide, leading to the formation of the alkyl iodide product. The reaction is typically favored for primary alkyl halides and methyl halides due to the better leaving group ability of iodide compared to other halogens. Additionally, the choice of solvent and reaction conditions can influence the rate and efficiency of the reaction.
The Finkelstein reaction finds numerous applications in organic synthesis and chemical transformations due to its ability to convert alkyl halides into alkyl iodides. Some key applications include:
- Synthesis of Alkyl Iodides: The primary application of the Finkelstein reaction is in the synthesis of alkyl iodides. Alkyl iodides are highly reactive alkylating agents and are valuable intermediates in organic synthesis. The reaction allows for the conversion of less reactive alkyl chlorides or bromides into the more reactive alkyl iodides, which can then undergo further transformations.
- Functional Group Interconversion: The Finkelstein reaction facilitates the interconversion of functional groups in organic molecules. By selectively exchanging one halogen for another, it enables the introduction or modification of functional groups, allowing for the synthesis of a wide range of organic compounds.
- Cross-Coupling Reactions: Alkyl iodides prepared via the Finkelstein reaction serve as valuable substrates in cross-coupling reactions, such as Suzuki-Miyaura coupling and Stille coupling. These reactions involve the formation of carbon-carbon bonds between two different alkyl or aryl groups, enabling the synthesis of complex organic molecules.
- Preparation of Radiopharmaceuticals: In radiochemistry, the Finkelstein reaction is used for the introduction of radioactive iodine isotopes (such as iodine-125 or iodine-131) into organic molecules. This application is particularly important in the synthesis of radiopharmaceuticals for medical imaging and therapy, where alkyl iodides labeled with radioactive iodine isotopes are used as imaging agents or therapeutic agents for the treatment of various diseases.
- Purification of Alkyl Halides: The Finkelstein reaction can also be employed for the purification of alkyl halides. By exchanging less desirable halogens (e.g., chlorine or bromine) with iodine, the reaction enables the purification and isolation of specific alkyl halides, which may be required for subsequent synthetic steps or applications.
Overall, the Finkelstein reaction is a versatile tool in organic synthesis, enabling the preparation of alkyl iodides and facilitating various chemical transformations for the synthesis of diverse organic compounds, including pharmaceuticals, agrochemicals, and materials.








