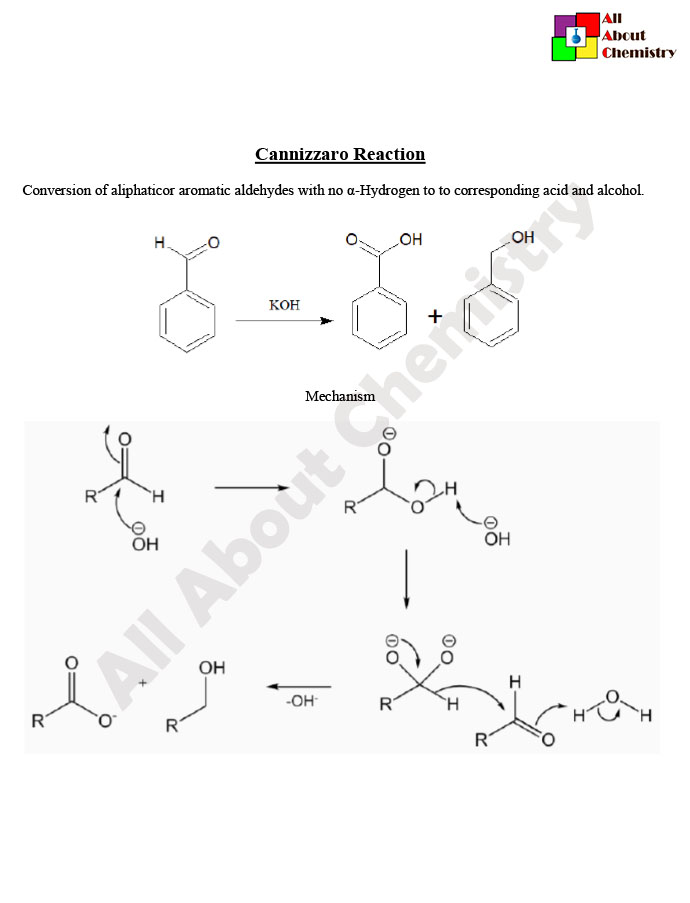
The Cannizzaro reaction, named after the Italian chemist Stanislao Cannizzaro, is a disproportionation reaction of an aldehyde into a carboxylic acid and an alcohol in the presence of a strong base, such as hydroxide ion (OH−). It is particularly notable because it is one of the few reactions where both oxidation and reduction occur simultaneously, without the use of an external oxidizing or reducing agent.
The general reaction scheme for the Cannizzaro reaction is as follows:
2 RCHO+OH−→RCOOH+RCH2OH
In this reaction, two molecules of the same aldehyde (RCHO) react in the presence of a base (OH−) to yield a carboxylic acid (RCOOH) and its corresponding alcohol (RCH2OH). One aldehyde molecule undergoes oxidation to form the carboxylic acid, while the other molecule is reduced to produce the alcohol.
The Cannizzaro reaction proceeds via a nucleophilic addition-elimination mechanism involving an alkoxide intermediate. The base (hydroxide ion) deprotonates one molecule of the aldehyde to generate the alkoxide ion (RCHO−). This alkoxide ion then attacks another molecule of the aldehyde, leading to the formation of a tetrahedral intermediate. This intermediate subsequently collapses to yield the carboxylic acid and alcohol products.
The Cannizzaro reaction is a disproportionation reaction involving an aldehyde molecule, which is oxidized to form a carboxylic acid, and another molecule of the same aldehyde, which is simultaneously reduced to yield an alcohol. The reaction typically occurs in the presence of a strong base, such as hydroxide ion (OH−). Below is the mechanism of the Cannizzaro reaction:
- Formation of Alkoxide Ion: The strong base (OH−) deprotonates one molecule of the aldehyde (RCHO), forming an alkoxide ion (RCHO−) and water (H2O).
- Nucleophilic Attack: The alkoxide ion (RCHO−) acts as a nucleophile and attacks another molecule of the aldehyde (RCHO), which is not deprotonated. This nucleophilic attack leads to the formation of a tetrahedral intermediate.
- Tetrahedral Intermediate Formation: The tetrahedral intermediate is formed as a result of the nucleophilic attack by the alkoxide ion on the carbonyl carbon of the aldehyde. This intermediate contains a new carbon-carbon bond.
- Hydrolysis of Tetrahedral Intermediate: The tetrahedral intermediate undergoes hydrolysis in the presence of water (H2O) or another molecule of the aldehyde. This step results in the cleavage of the carbon-carbon bond, leading to the formation of a carboxylic acid (RCOOH) and an alcohol (RCH2OH).
- Regeneration of Base: The carboxylic acid formed in the reaction can deprotonate another molecule of the aldehyde, regenerating the base (OH−) and completing the reaction cycle.
Overall, the Cannizzaro reaction proceeds through a series of nucleophilic addition and elimination steps, resulting in the simultaneous oxidation and reduction of two molecules of the same aldehyde. The reaction provides a convenient method for the conversion of aldehydes into carboxylic acids and alcohols under basic conditions.
The Cannizzaro reaction, with its unique ability to simultaneously oxidize and reduce aldehydes in the presence of a strong base, has several significant applications in organic synthesis. Here are some of its key uses:
- Preparation of Carboxylic Acids: One of the primary applications of the Cannizzaro reaction is in the synthesis of carboxylic acids from aldehydes. This transformation is particularly valuable for converting aldehydes that are difficult to oxidize using other methods into their corresponding carboxylic acids.
- Production of Alcohols: Simultaneous to the formation of carboxylic acids, the Cannizzaro reaction yields alcohols as well. This makes it a useful method for the preparation of primary alcohols from aldehydes.
- Sterically Hindered Aldehydes: The Cannizzaro reaction is effective even for sterically hindered aldehydes that may not undergo other reactions readily due to steric hindrance. It provides a convenient method for converting such aldehydes into useful carboxylic acids and alcohols.
- Regioselective Synthesis: In cases where an aldehyde has two different reactive sites (e.g., in certain aromatic aldehydes), the Cannizzaro reaction can exhibit regioselectivity, leading to the preferential formation of one product over the other. This regioselectivity can be valuable in synthetic planning.
- Synthesis of Fragrances and Flavor Compounds: The Cannizzaro reaction can be used in the synthesis of fragrance and flavor compounds. It allows for the conversion of aldehydes, which often contribute to the characteristic odor and taste of fragrances and flavors, into carboxylic acids and alcohols that are also common constituents of these compounds.
- Asymmetric Synthesis: Although the Cannizzaro reaction traditionally proceeds non-stereoselectively, asymmetric variants have been developed. These asymmetric Cannizzaro reactions utilize chiral catalysts or auxiliaries to induce stereoselectivity, enabling the synthesis of enantiomerically pure carboxylic acids and alcohols.
- Complex Molecule Synthesis: The Cannizzaro reaction can be incorporated as a step in the synthesis of complex molecules, allowing for the functionalization or transformation of specific carbonyl groups within the molecule.
Overall, the Cannizzaro reaction is a versatile tool in organic synthesis, offering a convenient method for the simultaneous oxidation and reduction of aldehydes to yield carboxylic acids and alcohols. Its broad applicability and ability to handle challenging substrates make it valuable in various fields of organic chemistry, including pharmaceuticals, fragrances, and fine chemicals synthesis.








