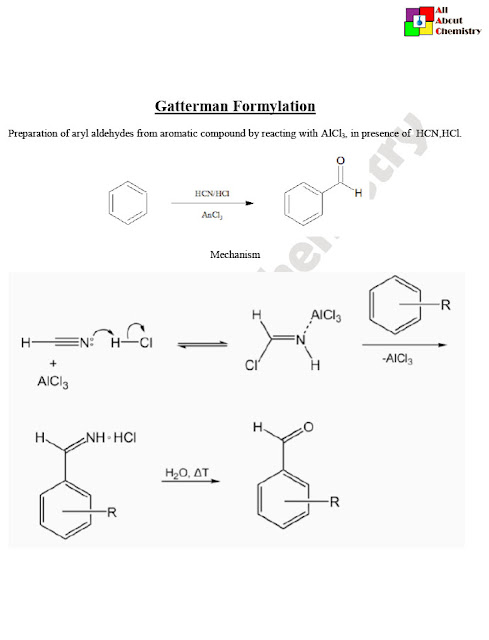
Gattermann formylation is a reaction used to introduce a formyl group (-CHO) onto aromatic compounds. It’s closely related to the Gattermann-Koch reaction, but instead of using carbon monoxide (CO) and hydrogen chloride (HCl), it utilizes carbon monoxide (CO) and hydrogen cyanide (HCN) in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3).
The general reaction scheme for Gattermann formylation is as follows:
Ar-H+CO+HCN+AlCl3→Ar-CHO
In this reaction, Ar-H represents an aromatic compound (such as benzene or substituted benzene), which reacts with carbon monoxide (CO) and hydrogen cyanide (HCN) in the presence of aluminum chloride (AlCl3) as a catalyst. The result is the introduction of a formyl group (-CHO) onto the aromatic ring, yielding an aromatic aldehyde.
The mechanism of the Gattermann formylation reaction involves the initial activation of carbon monoxide and hydrogen cyanide by the Lewis acid catalyst to form electrophilic species, followed by electrophilic aromatic substitution on the aromatic ring, resulting in the formation of an aryl cyanide intermediate. Subsequent hydrolysis or reduction of the aryl cyanide intermediate yields the aromatic aldehyde product.
Gattermann formylation has various applications in organic synthesis, including the preparation of aromatic aldehydes, which are important intermediates in the synthesis of pharmaceuticals, fragrances, and other fine chemicals. Additionally, aromatic aldehydes are commonly used as building blocks in organic synthesis for the construction of more complex molecules.
The mechanism of Gattermann formylation involves several steps and intermediates. Here’s a simplified mechanism for the conversion of an aromatic compound (Ar-H) into an aromatic aldehyde (Ar-CHO):
- Formation of the Acyl Cyanide Intermediate: Ar-H+CO+HCN+AlCl3→Ar-CO-CN. In the presence of the Lewis acid catalyst aluminum chloride (AlCl3), carbon monoxide (CO) and hydrogen cyanide (HCN) undergo activation to generate an electrophilic acyl cyanide intermediate (Ar-CO-CN). This step involves coordination of CO and HCN to aluminum chloride, followed by nucleophilic attack of the aromatic ring (Ar-H) on the coordinated CO to form the acyl cyanide.
- Protonation of the Acyl Cyanide: Ar-CO-CN+H3O+→Ar-CO-CNH2+The acyl cyanide intermediate is protonated by hydronium ion (H3O+) to form a positively charged intermediate (Ar-CO-CNH2+).
- Deprotonation to Form the Aryl Cyanide: Ar-CO-CNH2+→Ar-CO-CN+H2O. The positively charged intermediate undergoes deprotonation, facilitated by the presence of water (H2O), resulting in the regeneration of the aryl cyanide intermediate (Ar-CO-CN).
- Hydrolysis to Yield the Aromatic Aldehyde:Ar-CO-CN+H2O→Ar-CHO+NH3The aryl cyanide intermediate (Ar-CO-CN) undergoes hydrolysis in the presence of water (H2O) to yield the desired aromatic aldehyde (Ar-CHO) along with ammonia (NH3).
This mechanism outlines the key steps involved in the Gattermann formylation reaction, leading to the formation of aromatic aldehydes from aromatic compounds. The choice of Lewis acid catalyst and reaction conditions can influence the efficiency and selectivity of the reaction.
The Gattermann formylation reaction is a valuable tool in organic synthesis, offering a straightforward method for introducing formyl groups (-CHO) onto aromatic compounds. Here are some notable applications of Gattermann formylation:
- Synthesis of Aromatic Aldehydes: The primary application of Gattermann formylation is in the synthesis of aromatic aldehydes. These aldehydes serve as important intermediates in the preparation of various organic compounds, including pharmaceuticals, fragrances, dyes, and fine chemicals.
- Total Synthesis of Natural Products: Gattermann formylation plays a role in the total synthesis of natural products containing aromatic aldehyde functionalities. Chemists utilize this reaction as a key step in synthetic routes to access complex natural product scaffolds, enabling the study of their biological activities and structure-activity relationships.
- Functionalization of Aromatic Compounds: Gattermann formylation provides a convenient method for the functionalization of aromatic compounds, allowing chemists to modify their chemical properties and reactivity. The introduction of formyl groups onto aromatic rings expands the synthetic toolbox available for designing molecules with tailored functionalities.
- Pharmaceutical Synthesis: Aromatic aldehydes synthesized via Gattermann formylation are valuable building blocks in pharmaceutical synthesis. They serve as key intermediates for the preparation of drug candidates and pharmaceutical intermediates, enabling the synthesis of molecules with desired pharmacological properties.
- Fine Chemical Synthesis: Gattermann formylation finds applications in the synthesis of fine chemicals and specialty compounds used in various industrial sectors. The ability to introduce formyl groups onto aromatic rings provides a versatile route for the preparation of specialty chemicals, agrochemicals, and materials.
- Method Development and Optimization: Researchers continue to explore and optimize the Gattermann formylation reaction conditions and catalyst systems to improve efficiency, selectivity, and scalability. Novel applications and modifications of the reaction are investigated to expand its utility in organic synthesis.
Overall, Gattermann formylation offers a versatile and efficient method for the synthesis of aromatic aldehydes, with applications spanning across pharmaceuticals, natural product synthesis, fine chemicals, and materials science. Its simplicity and broad scope make it a valuable tool in the synthetic chemist’s toolbox.








