The Hass-Bender carbonyl synthesis is a method for the preparation of aldehydes and ketones from nitriles (RC≡N) using metal hydrides, such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4), and a protic acid, typically concentrated hydrochloric acid (HCl) or hydrobromic acid (HBr). This reaction provides a convenient way to introduce a carbonyl group into a molecule from a nitrile precursor.
Overall, the reaction can be represented as:
RC≡N+2H−+2H+→RC=NH2++H2
RC=NH2++2H2O→RCOH+NH3
where R represents an alkyl or aryl group.
The Hass-Bender carbonyl synthesis is a valuable tool in organic synthesis for the preparation of aldehydes and ketones from readily available nitrile starting materials. It offers a mild and efficient method for the introduction of carbonyl groups into organic molecules, allowing for the synthesis of a wide range of functionalized compounds.
The Hass-Bender carbonyl synthesis proceeds through two main steps: the reduction of the nitrile to an imine intermediate and the subsequent hydrolysis of this intermediate to form the aldehyde or ketone. Let’s break down the mechanism of each step:
Reduction of the Nitrile to an Imine Intermediate:
- The reaction begins with the nitrile (RC≡N) and a metal hydride reagent, such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4).The metal hydride reagent serves as a source of hydride ions (H^-). The hydride ion attacks the carbon atom of the nitrile, which is electrophilic due to the presence of the triple bond.This nucleophilic addition of the hydride ion to the nitrile carbon results in the formation of an imine intermediate (RC=NH). Simultaneously, the nitrogen atom receives a proton from the acidic medium.
RC−NH−+H+→RC=NH+H2
Hydrolysis of the Imine to the Aldehyde or Ketone:
- The imine intermediate formed in the first step undergoes hydrolysis under acidic conditions, typically in the presence of a protic acid like concentrated hydrochloric acid (HCl) or hydrobromic acid (HBr).In this step, a molecule of water (H2O) attacks the imine carbon, breaking the carbon-nitrogen double bond and forming a tetrahedral intermediate.This tetrahedral intermediate then collapses, expelling ammonia (NH3) and regenerating the carbonyl functionality, resulting in the formation of the aldehyde or ketone product.
RC=NH+H2O→Tetrahedral Intermediate
Tetrahedral Intermediate→RCHOH+NH3
RCHOH→RCHO
Overall, the Hass-Bender carbonyl synthesis involves the reduction of a nitrile to an imine intermediate followed by the hydrolysis of this intermediate to yield the desired aldehyde or ketone product. This reaction is a useful method for the conversion of nitriles into carbonyl compounds, offering a mild and efficient synthetic route.
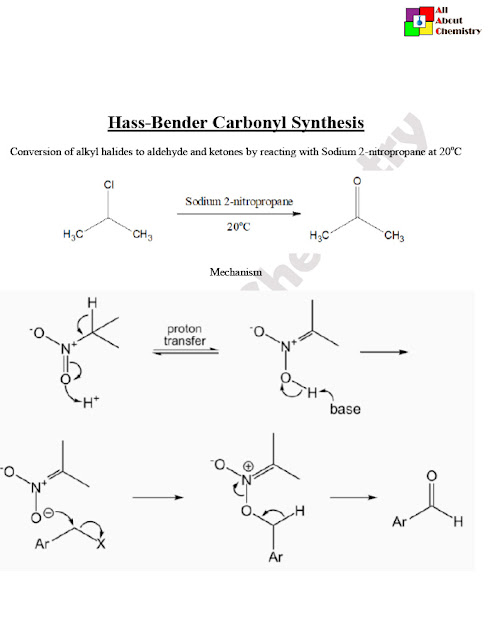
The Hass-Bender carbonyl synthesis has several important applications in organic synthesis due to its ability to convert nitriles into aldehydes or ketones. Some of the key applications include:
- Functional Group Interconversions: The Hass-Bender carbonyl synthesis provides a convenient method for converting nitrile functional groups into carbonyl groups (aldehydes or ketones). This transformation is valuable in organic synthesis for introducing carbonyl functionalities into molecules, enabling further manipulation and functionalization.
- Synthesis of Aldehydes and Ketones: The primary application of the Hass-Bender carbonyl synthesis is the preparation of aldehydes and ketones from nitrile precursors. Aldehydes and ketones are versatile intermediates used in a wide range of organic reactions, including aldol condensation, Grignard reactions, and Wittig reactions. Therefore, the ability to access these carbonyl compounds from nitriles expands the synthetic toolbox for organic chemists.
- Synthesis of Pharmaceutical Intermediates: Aldehydes and ketones obtained through the Hass-Bender carbonyl synthesis can serve as key intermediates in the synthesis of pharmaceuticals. Many pharmaceutical compounds contain carbonyl functionalities, making the synthesis of aldehydes and ketones from nitriles valuable for pharmaceutical research and development.
- Preparation of Fragrance and Flavor Compounds: Aldehydes and ketones are important components of fragrance and flavor compounds. The Hass-Bender carbonyl synthesis provides a route for the synthesis of these carbonyl compounds from nitrile starting materials, which can be further elaborated to produce a variety of fragrances and flavors used in perfumes, cosmetics, and food products.
- Industrial Applications: The Hass-Bender carbonyl synthesis may find applications in industrial processes where the conversion of nitriles into aldehydes or ketones is required. This synthesis offers a mild and efficient method for producing carbonyl compounds from readily available nitrile feedstocks, potentially providing cost-effective routes for large-scale chemical production.
Overall, the Hass-Bender carbonyl synthesis is a valuable synthetic method for the preparation of aldehydes and ketones from nitrile precursors, with applications ranging from pharmaceutical synthesis to fragrance and flavor production. Its versatility and efficiency make it an important tool in the repertoire of synthetic organic chemists.










