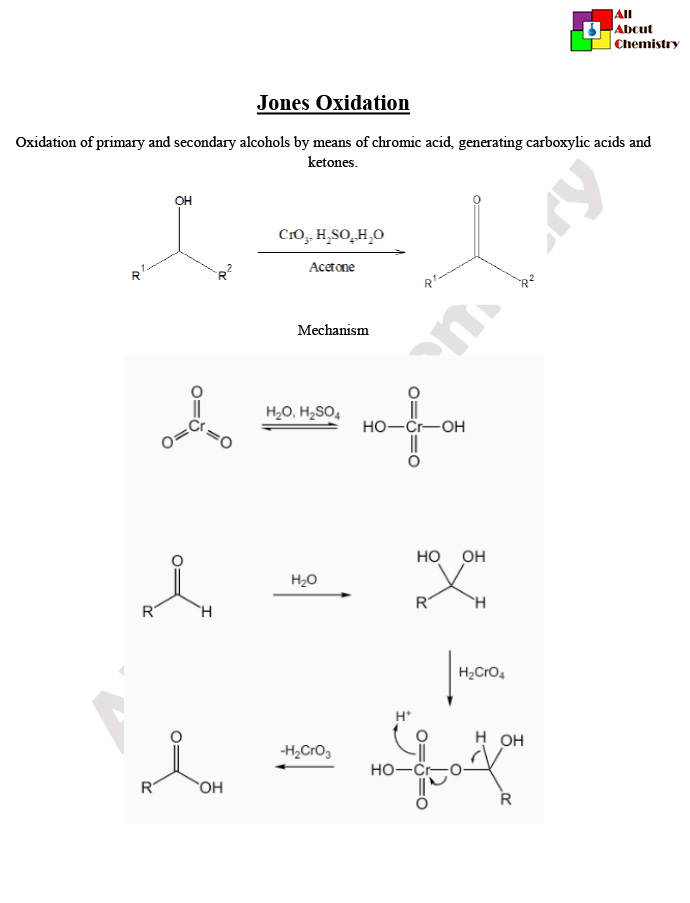
The Jones oxidation is a chemical reaction used to oxidize primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after the American chemist Sir Edward D. Jones who developed the reaction in the early 20th century. The reagent typically used in the Jones oxidation is a mixture of chromic trioxide (CrO3) and aqueous sulfuric acid (H2SO4) in acetone solvent.
The general equation for the Jones oxidation of a primary alcohol to a carboxylic acid is:
R−CH2OH+[O]→R−COOH+H2O
And for the oxidation of a secondary alcohol to a ketone:
R1−CH(OH)−R2+[O]→R1−CO−R2+H2O
In these equations, [O] represents the oxidizing agent provided by the Jones reagent.
The mechanism of the Jones oxidation involves several steps and intermediates. Here’s a simplified explanation of the mechanism for the oxidation of a primary alcohol to a carboxylic acid using the Jones reagent (a mixture of chromic trioxide and sulfuric acid in acetone):
- Formation of Chromate Ester: The primary alcohol reacts with chromic trioxide (CrO3) to form a chromate ester intermediate. This step involves the transfer of an oxygen atom from the chromic trioxide to the alcohol, resulting in the formation of a chromium-oxygen bond and the release of water.R−CH2OH+CrO3→R−CH2O−CrO3H+H2O
- Oxidative Cleavage: The chromate ester undergoes oxidative cleavage, leading to the formation of a carbonyl compound and a chromium(III) species. In the case of a primary alcohol, the result is the formation of a carboxylic acid. 3R−CH2O−CrO3H→R−COOH+Cr(OH)3
- Regeneration of Chromium(VI): The chromium(III) species produced in the previous step is oxidized back to chromium(VI) by another equivalent of chromic trioxide, regenerating the active oxidizing agent for further reactions.Cr(OH)3+CrO3→CrO3+H2O
Overall, the Jones oxidation converts a primary alcohol to a carboxylic acid by incorporating an oxygen atom from chromic trioxide into the alcohol functional group, followed by oxidative cleavage of the resulting chromate ester intermediate. This mechanism highlights the role of chromic trioxide as both the oxidizing agent and the source of the oxygen atom incorporated into the product.
The Jones oxidation is a widely used method in organic synthesis for converting primary and secondary alcohols into carboxylic acids and ketones, respectively. Its applications are diverse and include:
- Synthesis of Carboxylic Acids: The Jones oxidation is particularly useful for the conversion of primary alcohols to carboxylic acids. Carboxylic acids are important intermediates in organic synthesis and are found in various natural products and pharmaceuticals. The Jones oxidation provides a straightforward method for introducing carboxylic acid functional groups into organic molecules.
- Functional Group Interconversion: The conversion of primary alcohols to carboxylic acids and secondary alcohols to ketones allows for the interconversion of different functional groups. This can be valuable in designing synthetic routes for target molecules where specific functional groups need to be introduced or modified.
- Protection and Deprotection Strategies: In multi-step organic synthesis, it is often necessary to protect certain functional groups to prevent undesired reactions. The Jones oxidation can be used as a deprotection step to remove protecting groups that were installed on alcohols. For example, primary alcohols protected as acetals or ethers can be selectively oxidized to carboxylic acids, while leaving other functional groups intact.
- Natural Product Synthesis: Many natural products contain carboxylic acid or ketone functional groups. The Jones oxidation provides a useful tool for the synthesis of these natural products by allowing chemists to introduce these functional groups into precursor molecules.
- Industrial Applications: The Jones oxidation has industrial applications, particularly in the production of certain chemicals where carboxylic acids or ketones are desired intermediates. Additionally, it may be used in analytical chemistry for the quantification or identification of alcohols.
Despite its widespread use, it’s worth noting that the Jones oxidation has limitations, including potential over-oxidation and the toxicity associated with chromium compounds. As a result, alternative oxidation methods such as Swern oxidation and PCC oxidation are often preferred, especially in situations where milder reaction conditions or higher selectivity are desired.










