Chemicals in medicines can take various forms, including small organic molecules, peptides, proteins, and nucleic acids. Each class of compound offers unique advantages and challenges in terms of synthesis, stability, and pharmacokinetics. Furthermore, the development of pharmaceuticals involves rigorous testing to ensure efficacy, safety, and regulatory compliance before they reach the market.Chemicals in medicine are compounds used to diagnose, treat, or prevent diseases. They can be natural or synthetic and have varying mechanisms of action.
[We will provide some interesting Chemistry Research topics , this will help you to gain more knowledge about different spheres of chemistry. CLICK ]
Introduction
The principles of chemistry have been used for the benefit of mankind. Think of cleanliness — the materials like soaps, detergents,household bleaches, tooth pastes, etc. will come to your mind. Look towards the beautiful clothes — immediately chemicals of the synthetic fibres used for making clothes and chemicals giving colours to them will come to your mind. Food materials — again a number of chemicals about which you have learnt in the previous Unit will appear in your mind. Of course, sickness and diseases
remind us of medicines — again chemicals. Explosives, fuels, rocket propellents, building and electronic materials, etc., are all chemicals. Chemistry has influenced our life so much that we do not even realize that we come across chemicals at every moment; that
we ourselves are beautiful chemical creations and all our activities are controlled by chemicals.

Medicine is the field of health and healing. It includes nurses, doctors, and various specialists. It covers diagnosis, treatment, and prevention of disease, medical research, and many other aspects of health.Contemporary medicine applies biomedical sciences, biomedical research, genetics, and medical technology to diagnose, treat, and prevent injury and disease, typically through pharmaceuticals or surgery, but also through other diverse therapies.
Drugs
Drugs are chemicals of low molecular masses (~100 – 500u). These interact with macromolecular targets and produce a biological response. When the biological response is therapeutic and useful, these chemicals are called medicines and are used in diagnosis, prevention and treatment of diseases. Most of the drugs used as medicines are potential
poisons, if taken in doses higher than those recommended. Use of chemicals for herapeutic effect is called chemotherapy.The term ‘chemotherapy’ is used for the chemicals that are used for therapeutic effects. Research is constantly intensifying our understanding of medicinal Chemistry and leading to discoveries in this field. Based on the effects of medicines on biological systems, they are divided into different classes: antacids, antihistamines, analgesics, antiseptics, etc.Drugs are chemical substances of low molecular masses. They produce a biological response by interacting with macromolecular targets. When the biological response of these drugs is therapeutic and desirable, these chemicals are known as medicines and are used in the field of medicines that have helped doctors cure many diseases and save lives to a great extent. That has increased the longevity of people and provided us with a healthy and quality life as a boon!
Classification of Drugs
Drugs can be classified mainly on criteria outlined as follows:
(a) On the basis of pharmacological effect
This classification is based on pharmacological effect of the drugs. It is useful for doctors because it provides them the whole range of drugs available for the treatment of a articular type of problem. For example, analgesics have pain killing effect, antiseptics kill or arrest the growth of microorganisms.
(b) On the basis of drug action
It is based on the action of a drug on a particular biochemical process. For example, all antihistamines inhibit the action of the compound, histamine which causes inflammation in the body. There are various ways in which action of histamines can be blocked.
(c) On the basis of chemical structure
It is based on the chemical structure of the drug. Drugs classified in this way share common structural features and often have similar pharmacological activity.
(d) On the basis of molecular targets
Drugs usually interact with biomolecules such as carbohydrates, lipids,
proteins and nucleic acids. These are called target molecules or drug
targets. Drugs possessing some common structural features may have
the same mechanism of action on targets. The classification based on
molecular targets is the most useful classification for medicinal chemists.
Mechanism
Macromolecules of biological origin perform various functions in the body. For example, proteins which perform the role of biological catalysts in the body are called enzymes, those which are crucial to communication system in the body are called receptors. Carrier proteins carry polar molecules across the cell membrane. Nucleic acids have coded genetic information for the cell. Lipids and carbohydrates are structural parts of the cell membrane. We shall explain the drug-target interaction with the examples of enzymes and receptors.
(a) Catalytic action of enzymes For understanding the interaction between a drug and an enzyme, it is important to know how do enzymes catalyse the reaction .In their catalytic activity, enzymes perform two major functions: (i) The first function of an enzyme is to hold the substrate for a chemical reaction. Active sites of enzymes hold the substrate molecule in a suitable position, so that it can be attacked by the reagent effectively. Substrates bind to the active site of the enzyme through a variety of interactions such as ionic bonding, hydrogen bonding, van der Waals interaction or dipole-dipole interaction (ii) The second function of an enzyme is to provide functional groups that will attack the substrate and carry out chemical reaction.

(b) Drug-enzyme interaction Drugs inhibit any of the above mentioned activities of enzymes. These can block the binding site of the enzyme and prevent the binding of substrate, or can inhibit the catalytic activity of the enzyme. Such drugs are called enzyme inhibitors. Drugs inhibit the attachment of substrate on active site of enzymes in two different ways; (i) Drugs compete with the natural substrate for their attachment on the active sites of enzymes. Such drugs are called competitive inhibitors (ii) Some drugs do not bind to the enzyme’s active site. These bind to a different site of enzyme which is called allosteric site. This binding of inhibitor at allosteric site changes the shape of the active site in such a way that substrate cannot recognise it. If the bond formed between an enzyme and an inhibitor is a strong covalent bond and cannot be broken easily, then the enzyme is blocked permanently. The body then degrades the enzyme-inhibitor complex and synthesises the new enzyme. Receptors are proteins that are crucial to body’s communication process. Majority of these are embedded in cell membranes . Receptor proteins are embedded in the cell membrane in such a way that their small part possessing active site projects out of the surface of the membrane and opens on the outside region of the cell membrane In the body, message between two neurons and that between neurons to muscles is communicated through certain chemicals. These chemicals, known as chemical messengers are received at the binding sites of receptor proteins. To accommodate a messenger, shape of the receptor site changes. This brings about the transfer of message into the cell. Thus, chemical messenger gives message to the cell without entering the cell There are a large number of different receptors in the body that interact with different chemical messengers. These receptors show selectivity for one chemical messenger over the other because their binding sites have different shape, structure and amino acid composition. Drugs that bind to the receptor site and inhibit its natural function are called antagonists. These are useful when blocking of message is required. There are other types of drugs that mimic the natural messenger by switching on the receptor, these are called agonists. These are useful when there is lack of natural chemical messenger.
Antacids
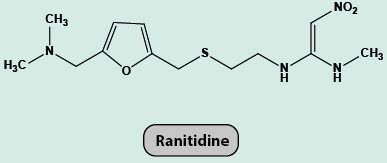
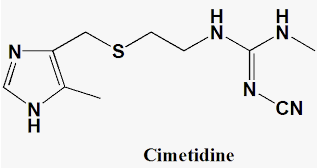
Over production of acid in the stomach causes irritation and pain. In severe cases, ulcers are developed in the stomach. Until 1970, only treatment for acidity was administration of antacids, such as sodium hydrogencarbonate or a mixture of aluminium and magnesium hydroxide. However, excessive hydrogencarbonate can make the stomach alkaline and trigger the production of even more acid. Metal hydroxides are better alternatives because of being insoluble, these do not increase the pH above neutrality. These treatments control only symptoms, and not the cause. Therefore, with these metal salts, the patients cannot be treated easily. In advanced stages, ulcers become life threatening and its only treatment is removal of the affected part of the stomach. A major breakthrough in the treatment of hyperacidity came through the discovery according to which a chemical, histamine, stimulates the secretion of pepsin and hydrochloric acid in the stomach. The drug cimetidine (Tegamet), was designed to prevent the interaction of histamine with the receptors present in the stomach wall. This resulted in release of lesser amount of acid. The importance of the drug was so much that it remained the largest selling drug in the world until another drug, ranitidine (Zantac), was discovered.
Antacids are over-the-counter (OTC) medications that help neutralize stomach acid.They work differently from other acid reducers such as H2 receptor blockers and proton pump inhibitors (PPIs). Those drugs work by reducing or preventing the secretion of stomach acid.Antacids can be used to treat symptoms of excess stomach acid, such as:
- acid reflux, which can include regurgitation, bitter taste, persistent dry cough, pain when lying down, and trouble swallowing
- heartburn, which is a burning sensation in your chest or throat caused by acid reflux
- indigestion, which is pain in your upper gut that can feel like gas or bloating
Antacids usually come in the following drug forms:
- liquid
- chewable gummy or tablet
- tablet that you dissolve in water to drink

Popular antacid brands include:
Antacids are typically safe for most people. However, people with certain medical conditions should talk with their doctors before taking certain antacids that contain aluminum hydroxide and magnesium carbonate.For example, people with heart failure may have sodium restrictions to help decrease fluid buildup. However, antacids often contain a lot of sodium. These people should ask their doctor before using antacids.People with kidney failure may develop a buildup of aluminum after using antacids. This can lead to aluminum toxicity. People with kidney failure also tend to have problems with electrolyte balance. All antacids contain electrolytes, which could make electrolyte balance problems worse.Talk to your child’s doctor before giving your child antacids. Children don’t typically develop symptoms of excess stomach acid, so their symptoms could be related to another condition.
Side effects from antacids are rare. However, they can occur, even when you use them according to the directions.Antacids can either cause constipation or have a laxative effect. Some people have had allergic reactions. Antacids might also increase the risk of developing sensitivities to certain foods.Many of the side effects of antacids come from not taking them as directed.Many antacids — including Maalox, Mylanta, Rolaids and Tums — contain calcium. If you take too much or take them for longer than directed, you could get an overdose of calcium. Too much calcium can cause:
- nausea
- vomiting
- mental status changes
- kidney stones

Excess calcium can also lead to alkalosis. In this condition, your body doesn’t make enough acid to function properly.If you feel like you need to use a lot of an antacid for relief, that might be a sign of another condition. If you’ve taken an antacid according to the directions and haven’t gotten relief, talk to your doctor.Antacids can interfere with the function of other drugs. If you take other medications, check with your doctor or pharmacist before using antacids.Some antacids, such as Alka-Seltzer, contain aspirin. The Food and Drug Administration issued a safety alertTrusted Source about this type of antacid in June 2016. This alert was issued because of reports of serious bleeding related to aspirin-containing antacids.If you take another medication that increases your risk of bleeding, such as an anticoagulant or antiplatelet drug, you shouldn’t take these antacids.Be sure to talk to your doctor before taking aspirin-containing antacids if you:
Have a history of stomach ulcers or bleeding disorders, are older than 60 years old, drink three or more alcoholic drinks per day.Antacids can often relieve symptoms of excess stomach acid. However, sometimes these symptoms mean you have a more serious condition.It’s important that you know how to recognize these conditions and how to respond to them. An upset stomach could actually be gastroesophageal reflux disease (GERD) or a peptic ulcer.Antacids can only soothe, not cure, some of the symptoms of these conditions. If you have severe pain that doesn’t get better after using the recommended dosage of antacids for two weeks, call your doctor.Some heart attack symptoms can also mimic stomach pains. You may be having a heart attack if you have severe chest pain that lasts longer than two minutes with any of the following symptoms:
- lightheadedness
- shortness of breath
- pain that radiates to your arms, shoulders, or jaw
- neck or back pain
- vomiting or nausea

If you think you may be experiencing a heart attack, call 911 or your local emergency services.The chemical substances which neutralises the excess acid in gastric juice and raise pH to an appropriate level in stomach are called antacids.For example: – Baking soda, mixture of Al and Mg hydroxide are commonly used antacids.Generally liquids antacids are more effective than tablets because of more of surface area available for interaction and neutralisation acids.Milk is a weak antacid.By using excess hydrogen carbonate stomach will become more of alkaline and as a result more of acid production happens.So Metal hydroxides are used instead of them which are insoluble and don’t increase the pH value also.
But they will only treat the symptoms not the cause.A major breakthrough in the treatment of hyperacidity happened by the discovery of chemical known as histamine which stimulates the secretion of pepsin and hydrochloric acid in the stomach.The drug cimetidine (Tegamet) was designed to prevent the interaction of histamine with the receptors present in the stomach wall.This resulted in release of lesser amount of acid.The importance of the drug was so much that it remained the largest selling drug in the world until another drug, ranitidine (Zantac), was discovered.
Antihistamines
Antihistamines are a class of drugs commonly used to treat symptoms of allergies. These drugs help treat conditions caused by too much histamine, a chemical created by your body’s immune system. Antihistamines are most commonly used by people who have allergic reactions to pollen and other allergens. They are also used to treat a variety of other conditions such as stomach problems, colds, anxiety and more.

Your body protects you from many threats. Your ribs protect your heart and lungs from injury. Your skin protects your body from outside elements like sun, wind and bacteria that can cause disease and infections. Your eyelashes protect your eyes from debris. And your body’s internal protection system – your immune system – battles substances that enter your body that are deemed “foreign.”
An allergy occurs when your immune system overreacts to the “foreign” substance. In the case of an allergy, substances that are usually harmless and don’t bother some people, such as dust or animal dander, do bother you! Your body views these substances as “foreign,” which then triggers an overreaction by your body’s defense system that includes the release of histamine. The substances that trigger the overreaction are called allergens. The symptoms that result are called an allergic reaction.
Allergies are one of the most common chronic conditions in the world. Some 40 million to 50 million people in the United States have them.Histamine is an important chemical that has a role in a number of different bodily processes. It stimulates gastric acid secretion, plays a role in inflammation, dilates blood vessels, affects muscle contractions in the intestines and lungs and affects your heart rate. It also helps transmit messages between nerve cells and helps fluids move through blood vessel walls. Histamine is also released if your body encounters a threat from an allergen. Histamine causes vessels to swell and dilate, leading to allergy symptoms.The top eight most common things that can cause an allergic reaction in some people include:
- Food.
- Dust.
- Pollen.
- Pet dander, saliva or urine.
- Mold.
- Insect bites and stings.
- Latex.
- Certain medications/drugs.
Too much histamine, caused by your body being oversensitive and overreacting to an allergen, causes a variety of symptoms. Symptoms include:
- Congestion, coughing.
- Wheezing, shortness of breath.
- Tiredness (fatigue).
- Itchy skin, hives and other skin rashes.
- Itchy, red, watering eyes.
- A running or blocked nose, or sneezing.
- Insomnia.
- Nausea and vomiting.
An antihistamine is a prescription or over-the-counter medication that blocks some of what histamine does. “Anti” means against, so antihistamines are medicines that work against or block histamine.
Antihistamines are divided into two major subtypes. The first subtype is called H-1 receptor antagonists or H-1 blockers. This subtype of antihistamines is used to treat allergy symptoms. The second subtype is called H-2 receptor antagonists or H-2 blockers. They are used to treat gastrointestinal conditions, including gastroesophageal reflux disease [GERD] (also called acid reflux), peptic ulcers, gastritis, motion sickness, nausea and vomiting. The naming structure (H-1 and H-2) tells doctors and scientists the cell type the location of the histamine receptor that the antihistamine medication blocks.
The H-1 blocker subtype is further broken down into two groups — first-generation antihistamines and second-generation antihistamines.Just like the name implies, the first generation antihistamine were the first type approved by the Food and Drug Administration (FDA). They began to be approved in the United States in the 1930s and are still prescribed today.They work on histamine receptor in the brain and spinal cord along with other types of receptors. Most notable about this generation of antihistamines is that they cross the blood-brain barrier, which results in drowsiness.
Second-generation antihistamines were approved by the FDA and first came to market in the 1980s. The second-generation antihistamines do not cross the blood-brain barrier to the extent that first-generation do and therefore do not cause drowsiness at standard dosage levels. Second-generation antihistamines are considered to be safer than first generation antihistamines because they don’t cause drowsiness and interact with fewer drugs.There are many prescription and over-the-counter H-1 antihistamines. If you have allergies, you’re likely taking a H-1 antihistamine. A few examples of first-generation over-the-counter and prescription H-1 blockers include:

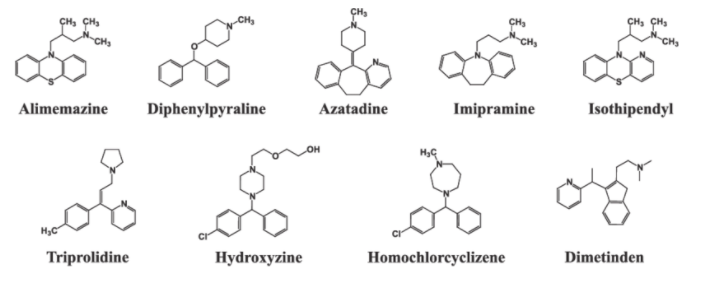
Brompheniramine (Children’s Dimetapp Cold®).Chlorpheniramine (Chlor-Trimeton®).Clemastine (Dayhist®).Cyproheptadine (Periactin®).Dexchlorpheniramine Dimenhydrinate (Dramamine®).Diphenhydramine (Benadryl®).Doxylamine (Vicks NyQuil®, Tylenol Cold and Couth Nighttime®).Hydroxyzine (Vistaril®).Phenindamine (Nolahist®).
A few examples of second-generation over-the-counter and prescription H-1 blockers include:
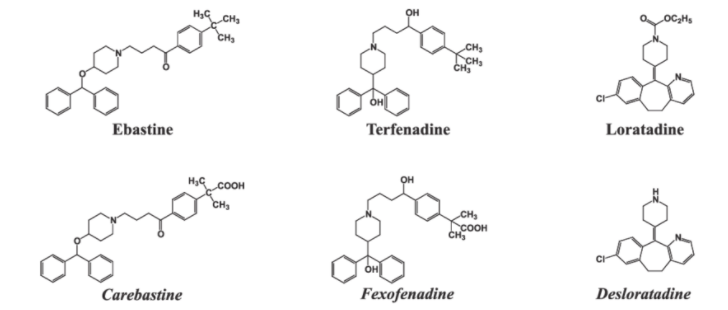
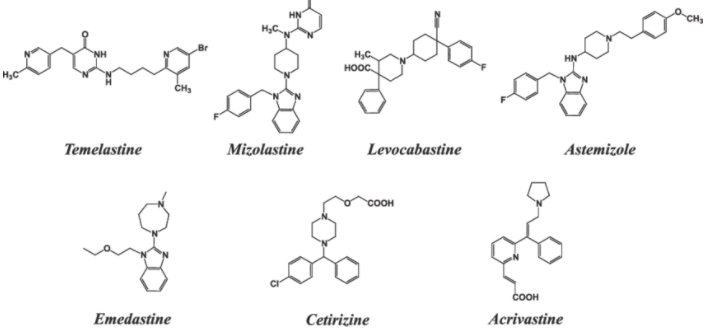
Azelastine (Astelin®).Loratadine (Claritin®).Cetirizine (Zyrtec®).Desloratadine (Clarinex®).Fexofenadine (Allegra®).
If you’re taking an antihistamine to help with stomach issues, you’re likely taking a H-2 antihistamine. A few examples of H-2 antihistamines include:
Cimetidine (Tagamet B®).Famotidine (Pepcid®).Nizatidine (Axid®).Ranitidine (Zantac®).
You and your healthcare provider should discuss specific antihistamines and decide together if the potential benefits of an antihistamine outweigh its potential side effects.
Some of the common side effects of first-generation antihistamines include:
Drowsiness.Dry mouth, dry eyes.Blurred or double vision.Dizziness and headache.Low blood pressure.Mucous thickening in the airways.Rapid heart rate.Difficulty urinating and constipation.
Some of the common side effects of second-generation antihistamines include:
Headache.Cough.Tiredness.Sore throat.Abdominal pain or discomfort,Nausea or vomiting.
Common side effects of H-2 antihistamines include:
Drowsiness.Joint or muscle pain.Headache.Confusion in the elderly.Dizziness.Breast swelling and tenderness.
Antihistamines come in several forms including:
Liquids.Lotions.Syrups.Gels.Eye drops.Tablets.Nasal sprays.Creams.Capsules.Suppositories.
Because there are so many antihistamine products, both over-the-counter and prescription, and because they are used to treat so many different conditions, you may need help figuring out which medication to take. For minor ailments, you can probably take over-the-counter products. You can read the package labeling and match your symptoms to the labeled symptoms. Also, never hesitate to ask the pharmacist. They are highly schooled in the actions and effects and side effects of drugs. You may need to try different antihistamines (but no more than one at a time unless directed by your physician) to find the best medication to manage your symptoms.

If you need a prescription antihistamine, you and your healthcare provider will work together to figure out what medication will be best for you. Many drugs interact with antihistamines, so your healthcare provider will want to know what medical conditions you have and medications you are currently taking. They will also want to know if you are pregnant, plan to become pregnant or are breastfeeding. Some antihistamines are not recommended in pregnancy because they may cause birth defects in very high doses. Antihistamines can pass into breast milk, so you should consult with your healthcare provider before using antihistamines if you are breastfeeding.
Children and the elderly are more sensitive to the effects of antihistamines, so special consideration will be given to the use of these products in these patients. Never give over-the-counter cough and cold antihistamines to children under four years of age. These medications can cause life-threatening side effects.
Histamine is a potent vasodilator. It has various functions. It contracts the smooth muscles in the bronchi and gut and relaxes other muscles, such as those in the walls of fine blood vessels. Histamine is also responsible for the nasal congestion associated with common cold and allergic response to pollen.Synthetic drugs, brompheniramine (Dimetapp) and terfenadine (Seldane), act as antihistamines. They interfere with the natural action of histamine by competing with histamine for binding sites of receptor where histamine exerts its effect.
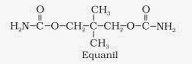
Tranquilizers
The term “tranquilizer” is used to encompass a wide variety of drugs that depress the central nervous system and have a calming effect. Most tranquilizers are prescription drugs, however, some can be purchased over-the-counter (OTC). They are used in the treatment of psychotic disorders, anxiety disorders, sleep disorders, and occasionally seizures or high blood pressure.
Common effects of tranquilizers include:
Relaxation,Sedation ,Induced drowsiness or sleep,Prevents or stops seizures,Relaxes muscles.
Major tranquilizers, also known as antipsychotic agents or neuroleptics, treat major mental disturbances in patients with schizophrenia and other psychotic disorders. Minor tranquilizers, also called antianxiety agents or anxiolytics, treat minor states of tension and anxiety in healthy individuals or patients with less severe mental disorders.
Benzodiazepines increase the work of the neurotransmitter gamma-aminobutyric acid (GABA). This helps reduce certain nerve-impulse transmissions. The result is reduced anxiety and a calming effect.Benzodiazepines, or “benzos,” are one of the most commonly abused prescription tranquilizers. Because of their widespread abuse and misuse, doctors only prescribe these types of tranquilizers to people with specific health problems, such as seizures. Benzos can cause health problems over-time, such as shallow breathing, paranoia, aggressive behavior, and other mental health problems.
Commonly prescribed “benzos” include:
- Alprazolam (Xanax)
- Diazepam (Valium)
- Oxazepam (Serax, Zaxopam)
- Clonazepam (Klonopin or Rivotril)
- Temazepam (Restoril)
- Lorazepam (Ativan)
- Chlordiazepoxide (Librium)
- Flurazepam (Dalmane)
People abuse opioids for pain relief and to feel their tranquilizing effects. However, narcotic drugs (which include opioids) are one of the leading causes of accidental, deadly overdoses. When opioids are mixed with benzos, this risk increases even more. If someone has built up a tolerance to a narcotic, they need to take a higher dose to feel the same effects.
Commonly abused narcotic pain pills include:
- Hydrocodone (Vicodin, Norco)
- Oxycodone (OxyContin, Roxicodone, Percocet)
- Hydromorphone (Dilaudid)
- Morphine (MS Contin)
- Fentanyl (Duragesic)
Barbiturates are sleep-inducing sedative drugs derived from barbituric acid. Commonly abused barbiturates include:
- Phenobarbital (Luminal)
- Secobarbital (Seconal)
- Amobarbital (Amynal)
- Pentobarbital (Nembutal)
“Hypnotic” medications (sleeping pills) are usually prescribed to people with insomnia. These medications act on the brain differently than the other tranquilizing drugs listed above.
Examples of sleep medications include:
- Zolpidem (Ambien)
- Triazolam (Halcion)
- Eszopiclone (Lunesta)
- Zaleplon (Sonata)
Not long after the popular drug Ambien was released, people began reporting sleepwalking cases on a widespread basis.By far, the most commonly abused sedating substance is alcohol (CNS depressant). When mixed with tranquilizer pills or inhaled or injected drugs, an increased level of sedation can occur, which kills many people every year.

Heroin is usually snorted or taken intravenously. Heroin is often mixed with another drug to “cut” the more expensive raw drug into cheaper portions. Common drugs combined with heroin include the highly sedating pharmaceutical drug fentanyl, an extremely potent narcotic often used to treat cancer-related pain.
Ketamine is a sedative normally used for moderate sedation in the hospital setting. Health professionals generally administer it as an intramuscular injection.
Although marijuana is now legal in many states in the US, it can cause a dangerous state of sedation when mixed with other substances. Edibles, or snacks made with high doses of potent strains of THC (the active narcotic in marijuana), can be easily dosed incorrectly.
A few potentially sedating over-the-counter (OTC) drugs are intended for insomnia, cold treatment, and other minor health issues.
Two common OTC sedating medicines include:
- Diphenhydramine (Benadryl)
- Dimenhydrinate (Dramamine)
These OTC drugs can both produce a significant sedating effect. Generally, they should not be combined with other tranquilizing medicines to prevent potential health and safety issues.
Kratom is an increasingly popular over-the-counter narcotic substitute that mimics the effects of opioids. In some states, such as Indiana, the use of Kratom is illegal.Benzodiazepines can produce potentially dangerous withdrawal symptoms when abused. Benzo withdrawal can cause seizures, panic attacks, insomnia, and muscle pain. Short acting Benzodiazepines, such as Xanax, have the most significant withdrawal symptoms.Opioids have a long list of unwanted side effects aside from respiratory depression, including constipation, which can cause the bowels to slow or stop normal digestion.
Other adverse side effects of tranquilizers include:
- Pruritic itching
- Sexual dysfunction
- Nausea
- Lowered appetite
- Seizures related to withdrawal
- Lowered cognition
- Short-term memory loss
- Irregular heart-rate
Taken alone, benzodiazepines pose a much lower risk of fatal overdose than when combined with opioids, even when misused. Because many opioid medications are combined with Acetaminophen (Tylenol, APAP), risk of serious liver damage and organ failure from overdose is an additional concern.Hypnotic class drugs, while effective for many who suffer from insomnia, can cause dangerous memory lapses. There have been cases of patients taking a normal dose of Ambien doing things like driving, shopping by phone or online, or preparing and eating full meals.There are many types of tranquilizers. Some carry more risk than others. In some cases, minor tranquilizers can be effective and safe when used under the supervision of a doctor.

However, it is essential to be aware of the adverse effects of some tranquilizers, particularly when it comes to benzodiazepines such as Xanax or Valium.These drugs can be just as dangerous as opioids, even though they are legal. For some individuals, it takes a few weeks to become addicted.If your doctor suggests a tranquilizer, it is essential to discuss the risks and benefits for your health. You should also always let your doctor know if you have a history of drug use. Your doctor may decide on a different type of treatment.
The clinical symptoms of physical dependency on tranquilizers include:
- Lowered inhibition
- Unusual happiness or euphoria
- Significant weight loss or gain (noticeable change in appearance)
- Bruises, swelling, and minor injuries related to falling while sedated
- Needle marks, bruising, and/or swelling caused by needle use
- History of “doctor shopping” to fuel their habit (having multiple pill bottles with different prescribing doctors names in their possession)
Benzodiazepine withdrawal is often associated with anxiety attacks, hand tremors, inability to concentrate, sweatiness, nausea, insomnia, seizure, and even psychosis.It is not generally a good idea to quit “cold turkey”, or stop using the drug abruptly. Life-threatening problems, such as seizures, breathing disturbances, and heart arrhythmias, could occur during the acute phase of withdrawal.Tranquilizers can lead to addiction over time, especially in long-term abuse cases. It is crucial to contact a medical practitioner or go to an emergency room to get immediate help during the withdrawal phase of the recovery process.
There are many addiction treatment options available to those who can afford it through insurance. If you cannot afford inpatient treatment, you may want to consider outpatient treatment. Both programs provide hands-on guidance and support while you work toward sobriety.There are also self-help programs that charitable organizations provide to nearly everyone. These programs include anonymous meetings, churches, and other support groups. Twelve-step programs, for example, are low-cost and available in most communities in the United States.
Analgesics
Analgesics are medications that relieve pain. Unlike medications used for anesthesia during surgery, analgesics don’t turn off nerves, change the ability to sense your surroundings or alter consciousness. They are sometimes called painkillers or pain relievers.
Analgesics are used to relieve pain and inflammation. For example:
- After surgery.
- Due to injury, such as a fractured bone.
- For acute (sudden, short-term) pain, such as a twisted ankle or headache.
- For aches and pains like menstrual cramps or muscle soreness.
- For chronic painful conditions such as arthritis, cancer or back pain.
There are two major groups of analgesics: anti-inflammatory analgesics and opioids. Anti-inflammatory drugs work by reducing inflammation (swelling) at the site of the pain. Examples include:

- Acetaminophen.
- Aspirin.
- COX inhibitors.
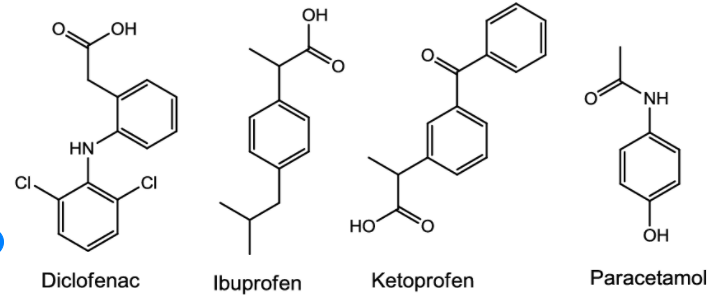
- Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen.
Analgesic opioids (also called narcotics) work by changing the brain’s perception of pain. An opioid can be any drug, natural or manmade. Many are similar to morphine, but newer, unrelated opioids have been created in the laboratory, too. Examples include:
Some pain medicines are available over the counter (OTC), which means you don’t need a prescription at all. Others are available by prescription only: often these include higher doses of OTC medications, combination analgesics and all opioids.Pain relievers are available in many forms, including:
- Films you place under the tongue to dissolve.
- Liquid you inject into your body with a syringe (needle).
- Liquid you swallow.
- Nasal spray that goes up the nose.
- Patches you place on the skin.
- Pills, tablets or capsules you swallow.
- Powder you mix and take various ways.
Anti-inflammatory analgesics are generally safe. But they can cause side effects and complications, if you use them too often, for too long or in very large doses:
- Damage to internal organs, such as the liver or kidneys.
- Diarrhea or constipation.
- Heart problems.
- Hypersensitivity response, which is like an allergic reaction.
- Nausea, upset stomach or heartburn.
- Ringing in the ears, or even deafness.
- Stomach ulcers.
- Trouble forming clots in the blood, which can lead to excessive bleeding.
Opioid analgesics can cause many of the same side effects and complications. Opioids are tightly controlled because they can cause physical dependence and are prone to abuse. The problem, which doctors now call substance abuse disorder, can be mild, moderate or severe, so it isn’t always recognized right away.
Some tell-tale signs a problem may be developing include:
- Often taking the medication in larger amounts than were intended.
- Unsuccessful efforts to cut down.
- Repeating failure to fulfill major obligations at work, school or home.
- Continued use despite having persistent problems.
- Giving up important social activities.
- Using even in dangerous situations (driving).
- Tolerance.
- Withdrawal.
Make sure you keep all pain relievers out of children’s reach.Tell your doctor about all medical conditions you have before taking an analgesic.Children shouldn’t take aspirin because a serious condition known as Reye’s syndrome can occur.Taking large amounts of Tylenol can harm your liver. Don’t take more than three grams (about six extra-strength pills, or nine regular pills) in a day.NSAIDs may increase your risk of stomach bleeding, heart attack, or stroke. Talk to your doctor about these risks.
If you take an opioid for a long time, you could develop a dependence as your body gets used to the drug. Some people also become addicted to opioids. Talk to your doctor if this is a concern.When you first start taking an opioid, you may need to avoid driving — or performing other tasks that require alertness — until you know how you react to the medicine.Tell your doctor about all prescription, non-prescription, illegal, recreational, herbal, nutritional, or dietary drugs you’re taking before starting on an analgesic.Follow the instructions on your prescription or package label carefully. Don’t take more of the medicine than is recommended.If you suspect an overdose of an analgesic, contact a poison control center or emergency room immediately.Analgesics reduce or abolish pain without causing impairment of consciousness, mental confusion, incoordination or paralysis or some other disturbances of nervous system. These are classified as follows:
(i) Non-narcotic (non-addictive) analgesics
(ii) Narcotic drugs
(i) Non-narcotic (non-addictive) analgesics: Aspirin and paracetamol belong to the class of non-narcotic analgesics. Aspirin is the most familiar example. Aspirin inhibits the synthesis of chemicals known as prostaglandins which stimulate inflammation in the tissue and cause pain. These drugs are effective in relieving skeletal pain such as that due to arthritis. These drugs have many other effects such as reducing fever (antipyretic) and preventing platelet coagulation. Because of its anti blood clotting action, aspirin finds use in prevention of heart attacks.

(ii) Narcotic analgesics: Morphine and many of its homologues, when administered in medicinal doses, relieve pain and produce sleep. In poisonous doses, these produce stupor, coma, convulsions and ultimately death. Morphine narcotics are sometimes referred to as opiates, since they are obtained from the opium poppy. These analgesics are chiefly used for the relief of postoperative pain, cardiac pain and pains of terminal cancer, and in child birth.
Antimicrobials
Diseases in human beings and animals may be caused by a variety of
microorganisms such as bacteria, virus, fungi and other pathogens.
An antimicrobial tends to destroy/prevent development or inhibit the
pathogenic action of microbes such as bacteria (antibacterial drugs),
fungi (antifungal agents), virus (antiviral agents), or other parasites
(antiparasitic drugs) selectively. Antibiotics, antiseptics and disinfectants
are antimicrobial drugs.
Antibiotics
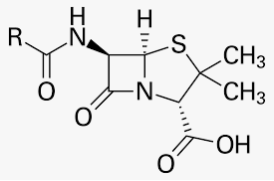
Antibiotics are powerful medicines that fight certain infections and can save lives when used properly. They either stop bacteria from reproducing or destroy them.Before bacteria can multiply and cause symptoms, the immune system can typically kill them. White blood cells (WBCs) attack harmful bacteria and, even if symptoms do occur, the immune system can usually cope and fight off the infection.Sometimes, however, the number of harmful bacteria is excessive, and the immune system cannot fight them all. Antibiotics are useful in this scenario.The first antibiotic was penicillin. Penicillin-based antibiotics, such as ampicillin, amoxicillin, and penicillin G, are still available to treat a variety of infections and have been around for a long time.
Several types of modern antibiotics are available, and they are usually only available with a prescription in most countries. Topical antibiotics are available in over-the-counter (OTC) creams and ointments.Some medical professionals have concerns that people are overusing antibiotics. They also believe that this overuse contributes toward the growing number of bacterial infections that are becoming resistant to antibacterial medications.According to the Centers for Disease Control (CDC), outpatient antibiotic overuse is a particular problem. Antibiotic use appears to be higher in some regionsTrusted Source, such as the Southeast.Use of carbapenems, a major class of last-line antibiotics, increased significantly from 2007 to 2010.There are different types of antibiotic, which work in one of two ways:
- A bactericidal antibiotic, such as penicillin, kills the bacteria. These drugs usually interfere with either the formation of the bacterial cell wall or its cell contents.
- A bacteriostatic stops bacteria from multiplying.
A doctor prescribes antibiotics for the treatment of a bacterial infection. It is not effective against viruses.Know whether an infection is bacterial or viral helps to effectively treat it.Viruses cause most upper respiratory tract infections (URTIs), such as the common cold and flu. Antibiotics do not work against these viruses.If people overuse antibiotics or use them incorrectly, the bacteria might become resistant. This means that the antibiotic becomes less effective against that type of bacterium, as the bacterium has been able to improve its defenses.A doctor can prescribe a broad-spectrum antibiotic to treat a wide range of infections. A narrow-spectrum antibiotic is only effective against a few types of bacteria.
Some antibiotics attack aerobic bacteria, while others work against anaerobic bacteria. Aerobic bacteria need oxygen and anaerobic bacteria do not.In some cases, a healthcare professional may provide antibiotics to prevent rather than treat an infection, as might be the case before surgery. This is the ‘prophylactic’ use of antibiotics. People commonly use these antibiotics before bowel and orthopedic surgery.
Antibiotics commonly cause the following side effects:
diarrhea, nausea, vomiting, rash, upset stomach, with certain antibiotics or prolonged use, fungal infections of the mouth, digestive tract, and vagina
Less common side effects of antibiotics include:
- formation of kidney stones, when taking sulphonamides
- abnormal blood clotting, when taking some cephalosporins)
- sensitivity to sunlight, when taking tetracyclines
- blood disorders, when taking trimethoprim
- deafness, when taking erythromycin and the aminoglycosides
Some people, especially older adults, may experience bowel inflammation, which can lead to severe, bloody diarrhea.In less common instances, penicillins, cephalosporins, and erythromycin can also cause inflamed bowels.
Antibiotics are used as drugs to treat infections because of their low toxicity for humans and animals. Initially antibiotics were classified as chemical substances produced by microorganisms (bacteria, fungi and molds) that inhibit the growth or even destroy microorganisms. The development of synthetic methods has helped in synthesising some of the compounds that were originally discovered as products of microorganisms. Also, some purely synthetic compounds have antibacterial activity, and therefore, definition of antibiotic has been modified. An antibiotic now refers to a substance produced wholly or
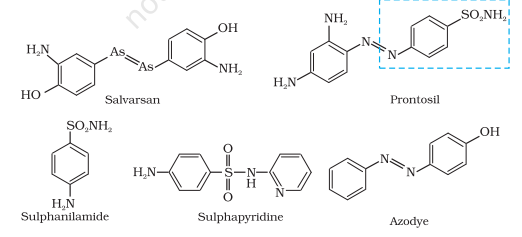
partly by chemical synthesis, which in low concentrations inhibits the growth or destroys microorganisms by intervening in their metabolic processes. The search for chemicals that would adversely affect invading bacteria but not the host began in the nineteenth century. Paul Ehrlich, a German bacteriologist, conceived this idea. He investigated arsenic based structures in order to produce less toxic substances for the treatment of syphilis. He developed the medicine, arsphenamine,known as salvarsan. Paul Ehrlich got Nobel prize for Medicine in 1908 for this discovery. It was the first effective treatment discovered for syphilis.
Although salvarsan is toxic to human beings, its effect on the bacteria, spirochete, which causes syphilis is much greater than on human beings. At the same time, Ehrlich was working on azodyes also. He noted that there is similarity in structures of salvarsan and azodyes. The –As = As– linkage present in arsphenamine resembles the –N = N – linkage present in azodyes in the sense that arsenic atom is present in place of nitrogen. He also noted tissues getting coloured by dyes selectively. Therefore, Ehrlich began to search for the compounds which resemble in structure to azodyes and selectively bind to bacteria.
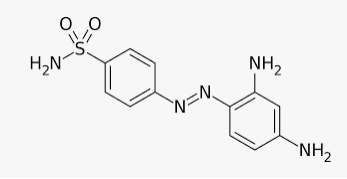
In 1932, he succeeded in preparing the first effective antibacterial agent, prontosil, which resembles in structure to the compound, salvarsan. Soon it was discovered that in the body prontosil is converted to a compound called sulphanilamide, which is the real active compound. Thus the sulpha drugs were discovered. A large range of sulphonamide analogues was synthesised. One of the most
effective is sulphapyridine. Despite the success of sulfonamides, the real revolution in
antibacterial therapy began with the discovery of Alexander Fleming in 1929, of the antibacterial properties of a Penicillium fungus. Isolation and purification of active compound to accumulate sufficient material for clinical trials took thirteen years. Antibiotics have either cidal (killing) effect or a static (inhibitory) effect on microbes.
The range of bacteria or other microorganisms that are affected by a certain antibiotic is expressed as its spectrum of action. Antibiotics which kill or inhibit a wide range of Gram-positive and Gram-negative bacteria are said to be broad spectrum antibiotics. Those effective mainly against Gram-positive or Gram-negative bacteria are narrow spectrum antibiotics. If effective against a single organism or disease, they are referred to as limited spectrum antibiotics. Penicillin G has a narrow spectrum.
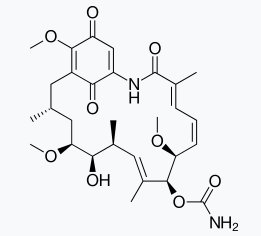
Ampicillin and Amoxycillin are synthetic modifications of penicillins. These have broad spectrum. It is absolutely essential to test the patients for sensitivity (allergy) to penicillin before it is administered. In India, penicillin is manufactured at the Hindustan Antibiotics in Pimpri
and in private sector industry. Chloramphenicol, isolated in 1947, is a broad spectrum antibiotic.It is rapidly absorbed from the gastrointestinal tract and hence can be given orally in case of typhoid, dysentery, acute fever, certain form of urinary infections, meningitis and pneumonia. Vancomycin and ofloxacin are the other important broad spectrum antibiotics. The antibiotic dysidazirine is supposed to be toxic towards certain
strains of cancer cells.
Antiseptics
Antiseptics are chemicals that people apply to the skin. They can reduce the number of microorganisms living on the skin, in wounds, and in mucous membranes.

Antiseptics are applied to the living tissues such as wounds, cuts,ulcers and diseased skin surfaces. Examples are furacine, soframicine, etc. These are not ingested like antibiotics. Commonly used antiseptic, dettol is a mixture of chloroxylenol and terpineol. Bithionol (the compound is also called bithional) is added to soaps to impart antiseptic properties.
Iodine is a powerful antiseptic. Its 2-3 per cent solution in alcoholwater mixture is known as tincture of iodine. It is applied on wounds. Iodoform is also used as an antiseptic for wounds. Boric acid in dilute aqueous solution is weak antiseptic for eyes.
Different types of antiseptic vary in cost, effectiveness, uses, and potential side effects.Healthcare workers often use antiseptics before carrying out medical procedures, such as drawing blood and performing surgery.Antiseptics are also available over the counter for cleaning and treating minor cuts. Some may also be suitable as a substitute for soap.

There are several types of antiseptics. Some are safe to use at home, whereas others are only suitable for use in clinical or hospital settings.Some common types of antiseptics include:
- alcohols, such as isopropyl alcohol and ethyl alcohol
- quaternary ammonium compound
- chlorhexidine and other diguanides, for use before operations
- antibacterial dye, to treat burns and wounds
- peroxide and permanganate, to disinfect the skin or to use as a mouthwash
- halogenated phenol derivative, in soaps and solutions
- quinolone derivative, which treats wounds and can be an ingredient in throat lozenges
Antiseptics have several potential uses. Some of the most common include:
- preventing infections on the skin, particularly for cuts, scrapes, or minor burns
- dry hand-washing, which healthcare workers may do between different procedures or patients
- cleaning the skin before a medical procedure, such as a blood draw or surgery
- treating throat infections with mouthwashes or lozenges
- cleaning mucous membranes, to treat infections or before using a catheter
Disinfectants
Disinfectants are chemical agents applied to non-living objects in order to destroy bacteria, viruses, fungi, mold or mildews living on the objects. By definition, disinfectant formulas must be registered with the Environmental Protection Agency (EPA). The “active ingredient” in each disinfectant formula is what kills pathogens, usually by disrupting or damaging their cells. Active ingredients are usually aided by other ingredients with various purposes. For example, surfactants can be added to a disinfectant formula to provide consistent wetting on a surface or to help in cleaning.

Several broad categories of disinfectants are used in commercial and industrial facility maintenance. Below are several of the most common types. While not an exhaustive list, these cover the large majority used today. If you’d like more detailed information on the pros and cons of these types of disinfectants, reference Nyco’s Liquid Disinfectants 101 chart.
Of special note: When a pathogen like SARS-CoV-2 that causes COVID-19 is initially identified by experts, it is classified as an “emerging pathogen.” The EPA allows only certain disinfectants to be designated effective against emerging pathogens. Nyco has several disinfectants with this special claim. Read your disinfectant label to identify whether it has the emerging pathogen claim.
| Hydrogen Peroxide | When formulated as ready-to-use disinfectants, hydrogen peroxide-based products are viewed as being safe, green, and sustainable for the environment. This is because they break down into naturally-occurring elements of water and oxygen. H2O2 disinfectants tend to kill a broad spectrum of bacteria and viruses quickly, are mildly acidic, and are effective cleaners. Some RTU products such as Nyco HPX Hydrogen Peroxide Disinfectant Cleaner will kill the SARS-CoV-2 in as little as one minute. Users should use caution with concentrated hydrogen peroxide however, as it can be unstable and dangerous. |
| Quaternary Ammonium Compounds (Quats) | Quats are a top choice for disinfection in hospital and institutional settings because of their low cost and quick action against a wide range of microorganisms. Quats can be formulated with a variety of detergents to provide both cleaning and disinfecting ability. Nyco’s Sani-Spritz Spray RTU disinfectant is an example of a quat-based disinfectant with both cleaning power and broad spectrum kill claims for many common and dangerous bacteria and viruses (including emerging pathogens and SARS-CoV-2). |
| Chlorine Compounds | Kills an array of organisms including resistant viruses, and is highly recommended for cleaning bodily fluids. Chlorine-based disinfectants are inexpensive and have relatively quick kill times, however they can be corrosive and cause discoloration as well as irritation if not used as directed. Chlorine Sanitizer II is an example of a chlorine disinfectant, ideal for use in healthcare settings and food preparation processing. |
| Alcohols | When diluted in water, alcohols are effective against a wide range of bacteria, though higher concentrations are often needed to disinfect wet surfaces. The downsides are they evaporate quickly (and thus may not remain on the surface long enough to kill), they’re flammable, and they may not have organic soil tolerance claims, meaning they may not be effective when organic matter (blood, for example) is present. |
| Aldehydes | Very effective against the bacteria that cause Tuberculosis, yet they need a high part per million (ppm) ratio to be effective for disinfection. Some bacteria have developed a resistance to aldehydes, and have been found to cause asthma and other health problems. They can also leave greasy residue and must be in an alkaline solution. |
| Iodophors | Can be used for disinfecting some semi-critical medical equipment but they can stain surfaces and have an unpleasant odor (think Iodine). Idophors aren’t often used in facility maintenance anymore. |
| Phenolic Compounds | Effective against pathogenic bacteria including Mycobacterium tuberculosis as well as fungi and viruses, but also very toxic and corrosive, attacking surfaces while they attack the organisms on them. Some areas enforce disposal restrictions on Phenols. |
There are four primary considerations you should evaluate when choosing a disinfectant to best meet the needs of your facility. Answering these questions will give you a framework for helping determine the best product(s) to use in your organization.
For example, you may be highly concerned about Staphylococcus aureus Methicillin Resistant (MRSA). Some disinfectants are EPA approved as effective against this bacteria. Nyco® Uno is one such disinfectant. Keep in mind that pathogens can have multiple strains, and disinfectants are certified for specific strains. Uno is also effective against Staphylococcus aureus (CA-MRSA) Community Associated Methicillin Resistant. Depending on your industry and facility type – healthcare, education, long-term care, hospitality – you will have varying needs and requirements.
Again, disinfectant formulas are registered to kill specific pathogens in a specific amount of time, and they need to be wet on a surface the entire time to be actively working. Thirty seconds to five minutes might be a typical kill time. If a disinfectant needs 10 minutes though, be sure it will actually stay wet that long. Alcohol-based disinfectants may vey well evaporate before their required contact time. Read and follow all directions for use and rewetting if necessary.
As you learned earlier in this article, some categories of disinfectants are toxic, some stain, others are corrosive, yet others have an undesirable odor. Check toxicity and flammability ratings on products, as well as any personal protective equipment (PPE) recommendations for disinfectants you apply. Be sure a disinfectant will not damage any surface it is intended for.

Some applications require multiple steps that may not always be feasible. Water hardness is one factor that can impact the effectiveness of some disinfectant formulas. Sani-Spritz Spray cleans and disinfects in just one step, making it a top choice for an easy, ready-to-use use disinfectant that addresses a broad spectrum of bacteria, viruses, fungi and mildew in hospitals, institutions, and industry.
Sorting through information about the various types of disinfectants takes time, but it’s a critical step to ensure you are making the best maintenance decision for your facility. Having the right products on hand along with a solid plan to prevent disease and infection will save effort and expense down the road, and give added peace of mind to you, your staff and any visitors that come through your doors.
In an analysis of the action of a disinfectant, it may often be difficult to distinguish
between the primary stage (characteristic of the mode of action) and the secondary
stage (merely a consequence of the action).
Action on the external membrane of the bacterial wall
A bacterium is protected from its environment by a membrane, the integrity of which
is essential to survival of the bacterium. This membrane consists of basic compounds
such as phospholipids and lipopolysaccharides, and is stabilised by Mg + +
and Ca + + cations. Thus, if ionised disinfecting molecules are absorbed or repelled by electrical charges at the initial contact and absorption stage, the following means of action will theoretically be possible:
- non-polar molecules may dissolve and enter the lipid phase
- specific carrying systems will lead other molecules through the membrane
- other molecules will be able to disturb the organisation of the membrane by
remaining bound to certain sites.
Action on the bacterial wall
The bacterial wall is important, as this confers rigidity and differs considerably
between Gram-positive and Gram-negative bacteria. This diversity leads to great
variation in the affinities of the hydrophilic disinfectants.
Action on the cytoplasmic membrane
An active molecule, such as a nutrient, may penetrate the cytoplasmic membrane in
the following ways:
a) passive diffusion (non-specific and slow)
b) active transport (specific, enabling the accumulation of products in bacteria after
either transformation or binding to a membrane protein).
Action on energy metabolism
Some disinfectants acting on adenosine triphosphatase (ATP) production are
studied below (in the section Action of various disinfectants’).
Action on the cytoplasm and nucleus
The disinfectant mechanism may operate on the cytoplasm and nucleus at the
chromosome level.
Action on bacterial spores
The impermeability and the presence of dipicolinic acid in bacterial spores make
these forms much more resistant to disinfectants than vegetative forms. The active
disinfectants include highly oxidising products, such as hydrogen peroxide and chlorine,
which can destabilise this structure in spores.

The elucidation of the exact mechanism of action of a disinfectant against viruses is
mor e difficult than for action against bacteria. Nevertheless, many studies on the
susceptibility of viruses to chemical agents demonstrate that the following factors are
important in understanding this action:
- presence of lipids in the viruses
- size of the viruses.
Noll and Youngner (18) classified the viruses in three groups: - group A: lipid-containing viruses (e.g. Herpesviridae, Paramyxoviridae,
Orthomyxoviridae) - group B: small (20-30 ran), non-lipid viruses (e.g. Picornaviridae, Parvoviridae)
- group C: other non-lipid viruses (e.g. Adenoviridae, Reoviridae, Papovaviridae).
Over the last thirty years, many authors have reached the same conclusions – from
very different in vitro tests – regarding the susceptibility of viruses to chemical agents:
Klein and Deforest (11,12), Scott (24), Derbyshire and Arkells (7), Evans et al. (8) and
Maris (15,16) all observe that the presence of lipid in a virus is uniformly associated
with a high degree of susceptibility to all disinfectants; the absence of lipid and small size
are associated with resistance to lipophilic chemical agents.
Fifty disinfectants with lipophilic properties (QACs, homologues of phenols,
amphoterics, polymeric biguanides) are active against group A viruses and not against
group B. However, chlorine and iodine compounds, oxidising agents, some aldehydes
(glutaraldehyde) and strong acidic or alkaline agents are active against most viruses.
Antifertility Drugs
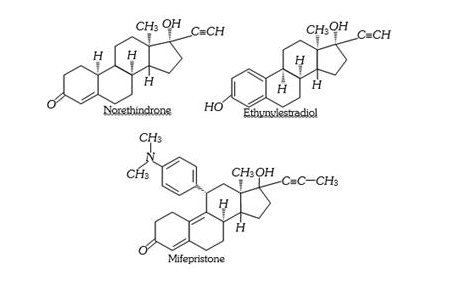
Antibiotic revolution has provided long and healthy life to people. The life expectancy has almost doubled. The increased population has caused many social problems in terms of food resources, environmental issues, employment, etc. To control these problems, population is required to be controlled. This has lead to the concept of family planning. Antifertility drugs are of use in this direction. Birth control pills essentially contain a
mixture of synthetic estrogen and progesterone derivatives. Both of these compounds are hormones. It is known that progesterone suppresses ovulation. Synthetic rogesterone derivatives are more potent than progesterone. Norethindrone is an
example of synthetic progesterone derivative most widely used as antifertility drug. The estrogen derivative which is used in combination with progesterone derivative is ethynylestradiol (novestrol).
Bibliography
- https://www.medicalnewstoday.com/
- https://pallipedia.org/medicine/
- https://www.healthline.com/health/antacids
- https://www.creative-enzymes.com/resource/catalytic-modes-of-enzymes_36.html
- https://my.clevelandclinic.org/health/drugs/21223-antihistamines
- https://www.researchgate.net/
- https://www.addictiongroup.org/drugs/other/tranquilizers/
- https://www.everydayhealth.com/analgesic/guide/
- https://my.clevelandclinic.org/
- https://www.medicalnewstoday.com/articles/10278
- https://ncert.nic.in/textbook/pdf/lech207.pdf
- https://www.embibe.com/exams/chemicals-in-medicines/
- https://www.medicalnewstoday.com/articles/antiseptic#uses
- https://www.nycoproducts.com/resources/blog/types-of-disinfectants-how-to-make-the-best-choice-for-your-facility/
- https://www.oie.int/doc/ged/D8965.PDF










