The greenhouse effect is a natural process that occurs when certain gases in the Earth’s atmosphere trap heat, causing global temperatures to rise.
The greenhouse effect is a natural process that plays a vital role in regulating the Earth’s temperature and making it suitable for life due the presence of green house gases. However, over the past century, human activities such as burning fossil fuels and deforestation have significantly increased the concentration of greenhouse gases in the atmosphere, leading to an intensification of the greenhouse effect. As a result, the planet’s temperature is rising at an alarming rate, causing a range of negative effects on the environment and human health. In this response, we will discuss the effects of the greenhouse effect in more detail.

Introduction
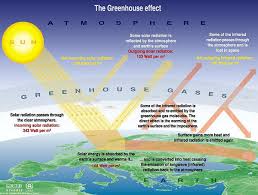
The greenhouse effect is a process that occurs after energy from a planet’s host star goes through the planet’s atmosphere and heats the planet’s surface. When the planet radiates the heat back out as thermal infrared radiation, greenhouse gases and clouds in the atmosphere absorb some of it. This traps the heat near the surface and reduces radiative cooling to space.
The Earth’s average surface temperature would be about −18 °C (−0.4 °F) without the greenhouse effect, compared to Earth’s 20th century average of about 14 °C (60 °F). In addition to naturally present greenhouse gases, burning of fossil fuels has increased amounts of carbon dioxide and methane in the atmosphere. As a result, global warming of about 1.2 °C (2.2 °F) has occurred since the industrial revolution, accelerating to a rate of 0.18 °C (0.32 °F) per decade more recently.
Greenhouse gases work by being transparent to wavelengths of radiation emitted by a star like the sun, but absorb wavelengths of radiation emitted by planets like the Earth. The wavelengths differ because matter radiates energy at a wavelength related to its temperature. The Sun is about 5,500 °C (9,930 °F), so it emits most of its energy in near infrared and visible wavelengths (as sunlight). The Earth’s surface temperatures are much lower, so it emits longer-wavelength thermal infrared radiation (radiated heat).

A runaway greenhouse effect occurs when greenhouse gases accumulate in the atmosphere through a positive feedback cycle to such an extent that they substantially block radiated heat from escaping into space, thus preventing the planet from cooling. A runaway greenhouse effect involving carbon dioxide and water vapor appears to have occurred on Venus. However, it is unlikely that human-caused greenhouse gas emissions alone could trigger a runaway effect on Earth.
The term greenhouse effect comes from an analogy to greenhouses. Both greenhouses and the greenhouse effect work by retaining heat from sunlight, but the mechanisms differ. Greenhouses primarily retain heat by preventing the movement of air (blocking convection), although their panels also limit heat radiation and conduction. The greenhouse effect only limits heat loss due to radiation; it has no impact on convection or conduction of heat.
Global warming describes the current rise in the average temperature of Earth’s air and oceans. Global warming is often described as the most recent example of climate change.
Earth’s climate has changed many times. Our planet has gone through multiple ice ages, in which ice sheets and glaciers covered large portions of Earth. It has also gone through warm periods when temperatures were higher than they are today.
Past changes in Earth’s temperature happened very slowly, over hundreds of thousands of years. However, the recent warming trend is happening much faster than it ever has. Natural cycles of warming and cooling are not enough to explain the amount of warming we have experienced in such a short time—only human activities can account for it. Scientists worry that the climate is changing faster than some living things can adapt to it.
In 1988, the World Meteorological Organization and the United Nations Environment Programme established a committee of climatologists, meteorologists, geographers, and other scientists from around the world. This Intergovernmental Panel on Climate Change (IPCC) includes thousands of scientists who review the most up-to-date research available related to global warming and climate change. The IPCC evaluates the risk of climate change caused by human activities.
According to the IPCC’s most recent report (in 2007), Earth’s average surface temperatures have risen about 0.74 degrees Celsius (1.33 degrees Fahrenheit) during the past 100 years. The increase is greater in northern latitudes. The IPCC also found that land regions are warming faster than oceans. The IPCC states that most of the temperature increase since the mid-20th century is likely due to human activities.
The factor that Earth has an average surface temperature pleasurably between the boiling
point and freezing point of water, therefore suitable for our kind of life, cannot be clarified by
merely proposing that planet Earth orbits at just the precise space from the sun to absorb just the right amount of solar radiation. The moderate temperatures are also the outcome of having just the precise kind of atmosphere. The atmosphere in planet Venus would produce hellish, Venuslike conditions on planet Earth; the Mars troposphere would leave earth shivering in a
Martian-type deep freeze. Additionally, parts of the earth’s atmosphere act as shielding blanket of just the right thickness, receiving appropriate solar energy to keep the global average temperature in an amusing range. The Martian blanket is too thin, and the Venusian
blanket is way too thick. The ‘blanket’ as stated here, is termed as a collection of atmospheric
gases called greenhouse gases based on the knowledge that the gases also capture heat
similar to the glass walls of a greenhouse. These gases, mostly water vapor, carbon
dioxide, methane, and nitrous oxide, all perform as effective global insulators. The conversation of inbound and outward-bound radiation that warms the Earth is often referred to as the greenhouse effect because a greenhouse works in much the same way.

Inbound Ultra Violet (UV) radiation easily passes through the glass walls of a greenhouse and is
absorbed by the plants and hard surfaces inside. Weaker Infrared (IR) radiation, however, has
difficulty passing through the glass walls and is trapped inside, that is, warming the greenhouse. This outcome lets tropical plants flourish inside a greenhouse, even during a cold winter.
The greenhouse influence upsurges the temperature of the Earth by trapping heat in our atmosphere. This retains the temperature of the Earth higher than it would be if direct heating by the Sun was the only source of warming. When sunlight reaches the surface of the Earth,
some of it is absorbed which warms the ground and some jumps back to space as heat. Most
Greenhouse gases that are in the atmosphere fascinate and then transmit some of this heat
back towards the Earth. The greenhouse effect is a foremost factor in keeping the Earth heartfelt because it keeps some of the planet’s heat that would otherwise escape from the atmosphere out to space. In fact, without the greenhouse effect the Earth’s average global temperature would be much colder and life on Earth as we recognize it would not be possible. The difference between the Earth’s actual average temperature 14°C (57.2°F) and the expected effective temperature just with the Sun’s radiation -19°C (-2.2°F) gives us the strength of the greenhouse effect, which is 33°C .
The greenhouse effect is a natural process that is millions of years old. It plays a critical role in a variable the overall temperature of the Earth. The greenhouse effect was first discovered by
Joseph Fourier in 1827, experimentally verified by John Tyndall in 1861, and quantified by
Svante Arrhenius in 1896 has published a paper on (A Synopsis on the Effects of
Anthropogenic Greenhouse Gases Emissions from Power Generation and Energy Consumption). It gives information about Despite the looming difficult energy context in the majority of countries in the world, global change in environmental dignity resulting from power generation and energy consumption scenario is rapidly becoming a globally disturbing phenomenon. The present study focused on the greenhouse effect: the greenhouse gases and their impacts on global warming.
Foundations of Greenhouse Effect
The greenhouse effect is mostly caused by the interaction of the sun’s energy with greenhouse
gases such as carbon dioxide, methane, nitrous oxide and fluorinated gases in the Earth’s
atmosphere. The ability of these gases to capture heat is what causes the greenhouse
effect .Greenhouse gases consist of three or more atoms. This molecular structure makes it
possible for these gases to trap heat in the atmosphere and then transfer it to the surface
which further warms the Earth. This uninterrupted cycle of trapping heat clues to an
overall increase in global temperatures. The procedure, which is very similar to the way a
greenhouse works, is the main reason why the gases that can produce this outcome are
collectively called as greenhouse gases .The prime forcing gases of the greenhouse effect
are: carbon dioxide (CO2), methane (CH4),nitrous oxide (N2O), and fluorinated gases.

Reaction Gas (Water vapor) of the Greenhouse Effect
Carbon dioxide is to some extent one of the greenhouse gases. It involves one carbon atom
with an oxygen atom bonded to each side. As soon as its atoms are bonded tightly together,
the carbon dioxide molecule can absorb infrared radiation and the molecule starts to vibrate.
Eventually, the vibrating molecule will emit the radiation again, and it will likely be absorbed by
yet another greenhouse gas molecule. This absorption-emission-absorption cycle serves to
keep the heat near the surface, effectively insulating the surface from the cold of space .
Carbon dioxide, water vapor (H2O), methane (CH4), nitrous oxide (N2O), and some limited
other gases are greenhouse gases. They all are molecules made up of more than two
constituents atoms, bound loosely enough together to be able to vibrate with the absorption
of heat. The foremost mechanisms of the atmosphere (N2 and O2) are two-atom molecules
too closely bound together to vibrate and consequently, they do not absorb heat and
subsidize to the greenhouse effect .Carbon dioxide, methane, nitrous oxide and the
fluorinated gases are all well-mixed gases in the atmosphere that do not react to changes in
temperature and air pressure, so the levels of these gases are not affected by condensation
effect. Water vapor also is a highly active component of the climate system that retorts
briskly to fluctuations in conditions by either dwindling into rain or snow or evaporating to
return to the atmosphere. Consequently, the imprint of the greenhouse effect is principally
circulated through water vapor, and it turns as a fast reaction effect. Carbon dioxide and the other non-condensing greenhouse gases are the vital gases within the Earth’s atmosphere that tolerate the greenhouse effect and rheostat its strength. Water vapor is a fast-acting feedback but its atmospheric concentration is controlled by the radiative forcing supplied by the non-condensing greenhouse gases. In fact, the greenhouse effect would collapse were it not for the presence of carbon dioxide and the other non-condensing greenhouse gases. Together the feedback by the condensing and the forcing by the non-condensing gases within the atmosphere both play an important role in the greenhouse effect.

Reduction of Greenhouse Gases
The primary objective of WWTPs is to meet effluent standards. In order to protect the receiving water body. However, reduction of GHG emissions from WWTPs requires a broadening in scope. The estimated quantity of N2O from WWTPs by the United States Environmental Protection Agency . Accounts for approximately 3% of N2O from all national sources which rank as the sixth largest contributor to GHG emissions. The right quantification of GHG is a necessity to better understand how to effectively reduce GHG emissions from WWTPs, as well as to improve
the accuracy in the GHG emission reporting processes. There is keen interest in climate change issues due to a fast increasing rate of GHG emissions. This has emphasized the need to inovate and establish right approaches to better design, control and optimize WWTPs on the plant-wide
scale. In recent years, one of the cheap modern and promising solutions to decreasing GHG emission into the Earth’s atmosphere is the employment bioremediation technique. Other mitigation plans to avert the negative outcomes of greenhouse effect may include activities such increase in tree planting, reduction in burning fossil fuels, exploitation of affordable, clean and renewable of energy, carbon dioxide capture and sequestration etc. Bioremediation technique employs microbial metabolism to remove pollutants. A bioremediation technique and strategy (phytoremediation enhanced by endophytic microorganisms) can be used to remove
hazardous waste including greenhouse gases from the biosphere. Phytoremediation is the most effective bioremediation technique employed to remove greenhouse gases. In hytoremediation, living green plants in situ are used. Living green plants have the ability to decrease or remove
contaminants from soil, air, water, and sediments. Recently, selected or engineered endophytic microorganisms have been used to improve the phytoremediation processes. Many studies have demonstrated the efficacy of endophytic microorganisms in accelerating these processes by interacting closely with their host plants .

Another technique for reducing the negative effects of the greenhouse effect is to use methanotrophic endophytes inhabiting Sphagnum Spp. which can act as a natural
methane filter. It can reduce CH4 and CO2 emission from peatlands by up to 50% .Studies have demonstrated potential ability of the plant–methanotrophic bacteria systems in the
reduction of methane emission up to 77%, depending on the season and the host plant .
Some Current Existing Challenges to Reducing Greenhouse Gases (GHG)
Currently, there are difficulty challenges in controlling GHG emissions for different WWTPs.
Measurement uncertainties and lack of transposable data still hinder a correct and required GHG emission quantification process .One recommendation to fill this gap includes the use of mathematical models which offer useful tools for assessing GHG and evaluating different mitigation alternatives before putting them into practice. GHG modelling can enhance the correct quantification of GHG emissions for different WWTP configurations and evaluate the
effects of various operating conditions. In recent years, a large portfolio of mathematical modelling studies has been developed to include GHG emissions during design, operation, and
optimization of WWTPs admonished the scientific community to examine the key elements of GHG modelling using a plant-wide approach. Several advantages and potentials of this approach include: i) an approach which takes into account the role of each plant treatment unit process and the interactions among them and ii) operation or
control of each particular unit, not only at local level but as a component of a system, and
avoids the risk of a sub-optimization (an example is a reduction of effluent quality at higher
operational costs.

The Solar Radiation
The sun radiates gigantic quantities of energy into space, crosswise a wide spectrum of wavelengths. Utmost of the radiant energy from the sun is concentrated in the visible and near-visible portions of the spectrum. The narrow band of visible light, between 400 and 700 nm, signifies 43% of the total radiant energy emitted. Wavelengths shorter than the visible account for 7 to 8% of the total, but are extremely important because of their high energy per photon. The shorter the wavelength of light, the more energy it contains. Accordingly, ultraviolet light is very energetic (accomplished by breaking apart stable biological molecules and instigating sunburn and skin cancers). The residual 49 – 50% of the radiant energy is spread over the wavelengths longer than those of visible light. These lie in the near infrared range from 700 to 1000 nm; the thermal infrared, between 5 and 20 microns; and the far infrared regions. Various components of earth’s atmosphere absorb ultraviolet and infrared solar radiation before it penetrates to the surface, but the atmosphere is quite transparent to visible light.
Absorbed by land, oceans, and vegetation at the surface, the visible light is transformed into heat and re-radiates in the form of invisible infrared radiation. During the day, earth heats up, but at night, all the accumulated energy would radiate back into space and the planet’s surface
temperature would fall far below zero very rapidly. The reason this doesn’t happen is that
earth’s atmosphere contains molecules that absorb the heat and re-radiate the heat in all
directions. This reduces the heat radiated out to space called greenhouse gases because they
serve to hold heat in like the glass walls of a greenhouse, these molecules are responsible for
the fact that the earth enjoys temperatures suitable for our active and complex biosphere.

Sources of Greenhouse Gas Emissions
In recent times, one of the major sources of greenhouse gas (GHG) emission is from water resource recovery facilities (wastewater treatment plants (WWTPs). Wastewater treatment plants (WWTPs) are recognized as one of the larger minor sources of GHG emissions . The WWTPs emit gases such as nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4). Increasing emission of GHG from this source poss harm to our climate .Biological mechanisms such as emissions of CO2 due to microbial respiration, emission of N2O by nitrification and denitrification, and emission of CH4 from anaerobic digestion processes are direct emissions from WWTPs. Sources that not regulated directly within the WWTP are indirect internal emission sources; consumption of thermal energy and indirect external emission sources; third-party biosolids hauling, chemical productions and their transportation to the plant, etc.
The increasing rate of GHG emissions is due to the changes in the economic output, extended
energy consumption, increasing emission from landfills, livestock, rice farming, septic rocesses,
and fertilizers as well as other factors. Increase industrialisation, use of fertilizers, burning of
fossil fuels and other human and natural activities result in a rise above normal average atmospheric temperature; thus posing threat to our environment. Research identifies methane and carbon dioxide as the main greenhouse gases . Therefore, the reduction of methane concentration in the atmosphere, both from natural and anthropogenic sources, is indispensable to tackle the negative outcomes of global warming.

Greenhouse Effect
Atmospheric scientists first used the word ‘greenhouse effect’ in the later 1800s. At that time, it was used to designate the naturally happening functions of trace gases in the atmosphere and did not have any negative implications. It was not up until the mid-1950s that the term greenhouse effect was attached to concern over climate alteration. And in contemporary decades, we often hear about the greenhouse effect in somewhat negative terms. The negative concerns are related to the possible impacts of an improved greenhouse effect. It is important to remember that without the greenhouse effect, lifecycle on earth as we know it would not be possible. While the earth’s temperature is reliant on upon the greenhouse-like action of the atmosphere, the extent of heating and cooling are toughly influenced by several factors just as
greenhouses are pretentious by various factors. In the atmospheric greenhouse effect, the type of surface that sunlight first happenstances are the most important factor. Forests, grasslands,
ocean surfaces, ice caps, deserts, and cities all absorb, reflect, and radiate radiation differently.
Sunlight falling on a white glacier surface strongly reflects back into space, resulting in minimal heating of the surface and lower atmosphere. Sunlight falling on a dark desert soil
is strongly absorbed, on the other hand, and contributes to significant heating of the surface
and lower atmosphere. Cloud cover also affects greenhouse warming by both reducing the
amount of solar radiation reaching the earth’s surface and by reducing the amount of radiation
energy emitted into space . Scientists outline the percentage of solar energy reflected back by a surface. Understanding local, regional, and global effects are life-threatening to foretelling global climate change.

Greenhouse Gases and Global Warming
Greenhouse gases (GHGs) such as carbon dioxide, methane, nitrous oxide, and halogenated compounds emissions are caused by human activities and some do occur naturally. The GHGs absorb infrared radiation and trap heat in the atmosphere, thereby enhancing the natural greenhouse effect defined as global warming. This natural occurrence warms the atmosphere and make life on earth possible, without which the low temperature will make life impossible to live on earth . “Gas molecules that captivate thermal infrared radiation, and are in a substantial amount, can force the climate system. These type of gas molecules are called greenhouse gases,” Michael Daley, an associate professor of Environmental Science at Lasell College told Live Science. Carbon dioxide (CO2) and other greenhouse gases turn like a blanket, gripping Infrared (IR) radiation and preventing it from evading into outer space. The net effect is the steady heating of Earth’s atmosphere and surface, and this process is called global warming. These greenhouse gases include water vapor, CO2, methane, nitrous oxide (N2O) and other gases. Since the dawn of the Industrial Revolution in the early 1800s, the scorching of fossil fuels like coal, oil, and gasoline have greatly increased the concentration of greenhouse gases in the atmosphere, specifically CO2, National Oceanic and Atmospheric Administration (NOAA). “Deforestation is the second largest anthropogenic basis of carbon dioxide to the atmosphere ranging between 6% and 17%,” said Daley. Some human activities like the production and consumption of fossil fuels, use of various chemicals agriculture, burning bush, waste from incineration processes and other industrial activities have increased the concentration of greenhouse gases (GHG), particularly CO2, CH4, and N2O in the atmosphere making them harmful.

This increase in atmospheric GHG concentration has led to climate change and global warming effect, which is motivating international efforts such as the Kyoto Protocol, signing of Paris Agreement on climate change and other initiatives to control negative outcomes of the greenhouse effect. The contribution of a greenhouse gas to global warming is commonly expressed by its global warming potential (GWP) which enables the comparison of global warming impact of the gas and that of a reference gas, typically carbon dioxide . Atmospheric CO2 intensities have increased by more than 40% since the beginning of the Industrial Revolution, from about 280 parts per million (ppm) in the 1800s to 400 ppm today. The last time Earth’s atmospheric levels of CO2 reached 400 ppm was during the Pliocene Epoch, between 5 million and 3 million years ago, according to the University of California, San Diego’s Scripps Institutions of Oceanography . The greenhouse effect, collective with growing levels of greenhouse gases and the resultant global warming, is expected to have profound consequences, according to the near-universal consensus of scientists . If global warming undergoes unimpeded, it will cause noteworthy climate change, a rise in sea levels, increasing ocean acidification, life threatening weather events and other severe natural and societal impacts, according to NASA, the Environmental Protection Agency(EPA) and other scientific and governmental bodies . Several scientists approve that the impairment of the Earth’s atmosphere and climate is long-gone the point of no reoccurrence or that the destruction is near the point of no return. “I agree that we have passed the point of avoiding climate change,” Josef Werne, an associate professor at the department of geology & planetary science at the University of Pittsburgh. In Werne’s opinion, there are three options from this point forward: 1. Do nothing and live with the moments. 2. Acclimatize to the changing climate (which includes things like rising sea level and related flooding). 3. Alleviate the impact of climate change by belligerently enacting policies that actually reduce the concentration of CO2 in the atmosphere. Keith Peterman, a professor of chemistry at York College of Pennsylvania, and Gregory Foy, an associate professor of chemistry at York College of Pennsylvania believes that the damage isn’t to that point yet and that international agreements and action can save the planet’s atmosphere 3. CONCLUSIONS The capacity of certain suggestion gases to be relatively transparent to inbound visible light from the sun, yet opaque to the energy radiated from the earth is one of the best silent procedures in the atmospheric sciences. This occurrence, the greenhouse effect, is what makes the earth a comfortable place for life’s activities. I recommend future work to be done on greenhouse gases.

Sources of greenhouse gases
Greenhouse gases have both natural and human-made sources. Natural sources of greenhouse gases include volcanic eruptions, geologic seeps, thawing permafrost, and natural wetlands1. Human activities since the Industrial Revolution have increased the atmospheric concentration of greenhouse gases, particularly carbon dioxide, by over 50%. The primary sources of human-made greenhouse gas emissions are electricity and heat production, agriculture, transportation, forestry, and manufacturing. The burning of fossil fuels, deforestation, and industrial processes are the main human-made sources of greenhouse gases. Methane emissions from landfills, coal mines, agriculture, and oil and natural gas operations, and nitrous oxide emissions from agricultural and industrial activities are also significant human-made sources of greenhouse gases.

The major constituents of Earth’s atmosphere, nitrogen (N2) (78%), oxygen (O2) (21%), and argon (Ar) (0.9%), are not greenhouse gases because molecules containing two atoms of the same element such as N2 and O2 have no net change in the distribution of their electrical charges when they vibrate, and monatomic gases such as Ar do not have vibrational modes. Hence they are almost totally unaffected by infrared (IR) radiation. Their IR interaction by way of collision-induced absorption is also small compared to the influences of Earth’s major greenhouse gases.
Greenhouse gases are those that absorb and emit infrared radiation in the wavelength range emitted by Earth. Carbon dioxide (0.04%), nitrous oxide, methane, and ozone are trace gases that account for almost 0.1% of Earth’s atmosphere and have an appreciable greenhouse effect.

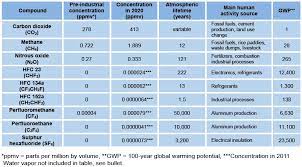
The most abundant greenhouse gases in Earth’s atmosphere, listed in decreasing order of average global mole fraction, are:
-
- Water vapor (H2O)
-
- Carbon dioxide (CO2)
-
- Methane (CH4)
-
- Nitrous oxide (N2O)
-
- Ozone (O3)
-
- Chlorofluorocarbons (CFCs and HCFCs)
-
- Hydrofluorocarbons (HFCs)
-
- Perfluorocarbons (CF4, C2F6, etc.), SF6, and NF3
Atmospheric concentrations are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound or absorption by bodies of water).The proportion of an emission remaining in the atmosphere after a specified time is the “airborne fraction” (AF). The annual airborne fraction is the ratio of the atmospheric increase in a given year to that year’s total emissions. As of 2006 the annual airborne fraction for CO2 was about 0.45. The annual airborne fraction increased at a rate of 0.25 ± 0.21% per year over the period 1959–2006.

Indirect radiative effects
Concentrations of carbon monoxide in April and October of 2000 in the lower atmosphere showing a range from about 50 parts per billion (blue pixels) to 220 parts per billion (red pixels) and 390 parts per billion (dark brown pixels).
Oxidation of CO to CO2 directly produces an unambiguous increase in radiative forcing although the reason is subtle. The peak of the thermal IR emission from Earth’s surface is very close to a strong vibrational absorption band of CO2 (wavelength 15 microns, or wavenumber 667 cm−1). On the other hand, the single CO vibrational band only absorbs IR at much shorter wavelengths (4.7 microns, or 2145 cm−1), where the emission of radiant energy from Earth’s surface is at least a factor of ten lower. Oxidation of methane to CO2, which requires reactions with the OH radical, produces an instantaneous reduction in radiative absorption and emission since CO2 is a weaker greenhouse gas than methane. However, the oxidations of CO and CH4 are entwined since both consume OH radicals. In any case, the calculation of the total radiative effect includes both direct and indirect forcing.
A second type of indirect effect happens when chemical reactions in the atmosphere involving these gases change the concentrations of greenhouse gases. For example, the destruction of non-methane volatile organic compounds (NMVOCs) in the atmosphere can produce ozone. The size of the indirect effect can depend strongly on where and when the gas is emitted.
Methane has indirect effects in addition to forming CO2. The main chemical that reacts with methane in the atmosphere is the hydroxyl radical (OH), thus more methane means that the concentration of OH goes down. Effectively, methane increases its own atmospheric lifetime and therefore its overall radiative effect. The oxidation of methane can produce both ozone and water; and is a major source of water vapor in the normally dry stratosphere. CO and NMVOCs produce CO2 when they are oxidized. They remove OH from the atmosphere, and this leads to higher concentrations of methane. The surprising effect of this is that the global warming potential of CO is three times that of CO2. The same process that converts NMVOCs to carbon dioxide can also lead to the formation of tropospheric ozone. Halocarbons have an indirect effect because they destroy stratospheric ozone. Finally, hydrogen can lead to ozone production and CH4 increases as well as producing stratospheric water vapor.

Role of water vapor
Increasing water vapor in the stratosphere at Boulder, Colorado
Water vapor accounts for the largest percentage of the greenhouse effect, between 36% and 66% for clear sky conditions and between 66% and 85% when including clouds. Water vapor concentrations fluctuate regionally, but human activity does not directly affect water vapor concentrations except at local scales, such as near irrigated fields. Indirectly, human activity that increases global temperatures will increase water vapor concentrations, a process known as water vapor feedback. The atmospheric concentration of vapor is highly variable and depends largely on temperature, from less than 0.01% in extremely cold regions up to 3% by mass in saturated air at about 32 °C.
The average residence time of a water molecule in the atmosphere is only about nine days, compared to years or centuries for other greenhouse gases such as CH
4 and CO2.Water vapor responds to and amplifies effects of the other greenhouse gases. The Clausius–Clapeyron relation establishes that more water vapor will be present per unit volume at elevated temperatures. This and other basic principles indicate that warming associated with increased concentrations of the other greenhouse gases also will increase the concentration of water vapor (assuming that the relative humidity remains approximately constant; modeling and observational studies find that this is indeed so). Because water vapor is a greenhouse gas, this results in further warming and so is a “positive feedback” that amplifies the original warming. Eventually other earth processes offset these positive feedbacks, stabilizing the global temperature at a new equilibrium and preventing the loss of Earth’s water through a Venus-like runaway greenhouse effect.

Contribution of clouds to Earth’s greenhouse effect
The major non-gas contributor to Earth’s greenhouse effect, clouds, also absorb and emit infrared radiation and thus have an effect on greenhouse gas radiative properties. Clouds are water droplets or ice crystals suspended in the atmosphere.
Schmidt et al. (2010) analyzed how individual components of the atmosphere contribute to the total greenhouse effect. They estimated that water vapor accounts for about 50% of Earth’s greenhouse effect, with clouds contributing 25%, carbon dioxide 20%, and the minor greenhouse gases and aerosols accounting for the remaining 5%. In the study, the reference model atmosphere is for 1980 conditions.The contribution of each gas to the greenhouse effect is determined by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, the direct radiative effect of a mass of methane is about 84 times stronger than the same mass of carbon dioxide over a 20-year time frame but it is present in much smaller concentrations so that its total direct radiative effect has so far been smaller, in part due to its shorter atmospheric lifetime in the absence of additional carbon sequestration. On the other hand, in addition to its direct radiative impact, methane has a large, indirect radiative effect because it contributes to ozone formation. Shindell et al. (2005) argues that the contribution to climate change from methane is at least double previous estimates as a result of this effect.
What is causing increased greenhouse gas concentrations?

Most greenhouse gases released in the United States contain carbon. Carbon naturally cycles throughout the planet and the air. There is carbon moving around “in circulation,” such as the CO2 we breath and carbon contained in plant and animal tissue. And there is carbon locked in “long-term storage”, called carbon sinks. Carbon in underground oil reserves or in trees that live hundreds of years are examples are carbon sinks. Many human activities take carbon out of carbon sinks and put it back into the atmosphere. Oil reserves that took millions of years to form are used up in decades. Forests that have stood for centuries are harvested or burned in a matter of weeks. Through these activities, we add more carbon dioxide to the atmosphere than can naturally be reabsorbed.
Efforts to slow or stop climate change revolve around righting the carbon imbalance in the atmosphere. This can be done by decreasing greenhouse gas emissions, for example by reducing fossil fuel use. Or it can involve increasing the amount of carbon being captured and stored in carbon sinks, a process called carbon sequestration.

Important sources of greenhouse gas emissions include:
-
- Burning fossil fuels, including oil, coal, and natural gas
-
- Producing and using industrial products
-
- Agriculture, including cows and some crops
-
- Destroying or disrupting ecosystems that act as carbon sinks
There are also natural sources of greenhouse gases, including volcanic eruptions, geologic seeps (such as hot springs and geothermal vents), thawing permafrost, and forest fires. Climate change and human activities can accelerate natural emissions. Warmer temperatures defrost permafrost and heat up oceans, releasing the carbon long stored in these systems. Wetlands drained for agriculture can rapidly switch from being carbon sinks to being carbon sources. And human ignitions and climate-driven dryness mean long, intense fire seasons, releasing billions of metric tons of carbon dioxide around the world each year.

Greenhouse Gas Emissions on Public Lands
The USGS conducts research on greenhouse gas emissions and carbon sequestration in public lands. Public lands maintained by the U.S. Department of the Interior make up about one-fifth of the Nation’s land area. They include national parks, seashores, and monuments managed by the National Park Service; national wildlife refuges managed by the U.S. Fish and Wildlife Service; and working lands and offshore mineral rights managed by the Bureau of Land Management.
In 2018, the USGS estimated the amount of carbon released by and sequestered in U.S. federal lands. (USGS Scientific Investigations Report 2018-5131). We found that about one-quarter of the United States’ emissions come from combustion of coal, oil, and gas extracted from public lands.
The USGS also investigates methods of land management aimed at decreasing emissions from federal lands. We provide decision-makers, local communities, and land managers with tools to analyze tradeoffs associated with changing energy practices. We also develop natural carbon dioxide removal technologies to remove carbon from the atmosphere through carbon sequestration and to decrease natural methane emissions.

WATER VAPOR
NCDC explains that changes in the concentration of water vapor result from climate feedbacks related to the warming of the atmosphere and not from activities related to industrialization. The feedback loop in which water is involved is critically important to projecting future climate change, NCDC continues, “but as yet is still fairly poorly measured and understood.” The agency continues:
As the temperature of the atmosphere rises, more water is evaporated from ground storage (rivers, oceans, reservoirs, soil). Because the air is warmer, the absolute humidity can be higher (in essence, the air is able to ‘hold’ more water when it’s warmer), leading to more water vapor in the atmosphere. As a greenhouse gas, the higher concentration of water vapor is then able to absorb more thermal infrared energy radiated from the Earth, thus further warming the atmosphere. The warmer atmosphere can then hold more water vapor and so on and so on. This is referred to as a ‘positive feedback loop’. However, huge scientific uncertainty exists in defining the extent and importance of this feedback loop. As water vapor increases in the atmosphere, more of it will eventually also condense into clouds, which are more able to reflect incoming solar radiation (thus allowing less energy to reach the Earth’s surface and heat it up). The future monitoring of atmospheric processes involving water vapor will be critical to fully understand the feedbacks in the climate system leading to global climate change. As yet, though the basics of the hydrological cycle are fairly well understood, we have very little comprehension of the complexity of the feedback loops. Also, while we have good atmospheric measurements of other key greenhouse gases such as carbon dioxide and methane, we have poor measurements of global water vapor, so it is not certain by how much atmospheric concentrations have risen in recent decades or centuries, though satellite measurements, combined with balloon data and some in-situ ground measurements, indicate generally positive trends in global water vapor.

CARBON DIOXIDE (CO2)
Let’s now consider what federal agency and academic researchers consider to be the “most important greenhouse gases.”
Carbon dioxide (not to be confused with carbon monoxide, CO, associated with vehicle tail pipe emissions or with home CO alerts) occurs both naturally and as a result of human activities. It is an inevitable byproduct of the combustion of fossil fuels, such as coal, gasoline, and natural gas. In 2013, CO2 accounted for about 82 percent of all U.S. greenhouse gas emissions from human activities. Citing data from the National Research Council’s 2011 Advancing the Science of Climate Change, the U.S. Environmental Protection Agency (EPA) website reports that “human activities are altering the carbon cycle—both by adding more CO2 to the atmosphere and by influencing the ability of natural sinks, like forests, to remove CO2 from the atmosphere. While CO2 emissions come from a variety of natural sources, human-related emissions are responsible for the increase that has occurred in the atmosphere since the industrial revolution.” Concentrations of CO2 in the atmosphere have increased by more than one-third since the beginning of the Industrial Age. Projections over coming years see that trend continuing.

Long stable in the range of about 280 parts per million (PPM) in the atmosphere, CO2 concentrations currently are more in the range of 400 PPM. The continuing upward trajectory of CO2 concentrations under what is called a “business as usual” scenario is one of the matters of particular concern to climate scientists.
It’s not so much the GWP of carbon dioxide that is concerning, but rather the current and projected continued growth in emissions and atmospheric concentrations, and the fact that CO2 is very long-lived – more than a century – in the atmosphere. What we emit today is going to remain in the atmosphere for a very, very long time.
Carbon dioxide is, of course, critical to plant growth and to food production, and it’s emitted each time we humans exhale. In the atmosphere, however, it’s a case of too much of a good thing: The science community has known since the research findings of Swedish scientist and Nobel Laureate Svante Arrhenius more than a century ago that humans’ burning of fossil fuels leads to a greenhouse effect caused by the release of CO2. For the science community, that’s “old hat” and widely accepted.

METHANE (CH4)
Methane, a hydrocarbon gas resulting from both natural causes and as a result of human activities such as agriculture and farming, is an especially potent (read “efficient,” but not as a compliment) GHG and absorber of radiation. Methane is far less abundant than CO2 in the atmosphere and it has a considerably shorter lifespan of 12 years. The National Research Council says that concentrations of methane in the atmosphere, while increasing sharply throughout the 1980s, have since leveled-off somewhat and now stand about two and one-half times their preindustrial levels.
Valued for energy production, methane, like CO2, is odorless and colorless – and it has both beneficial and harmful qualities.
EPA figures indicate that human activities account for over 60% of total methane emissions, primarily from industry, agriculture and waste management activities. This chart shows methane contributions by various sources:

According to the EPA web site, wetlands are the largest natural source of methane, emitting it from bacteria that decompose organic materials in the absence of oxygen. Smaller sources include termites, oceans, sediments, volcanoes and wildfires.
EPA reports that methane emissions in the United States decreased by nearly 11 percent between 1990 and 2012, during which time emissions “increased from sources associated with agricultural activities, while emissions decreased from sources associated with the exploration and production of natural gas and petroleum products.”
In recent years, some media reports have focused increased attention on the potential for sudden and massive releases of long-bottled-up methane and methane hydrates currently sequestered by frozen tundra. The concern is that melting of the Arctic tundra could lead to potentially catastrophic and abrupt releases of methane. An excellent resource for further understanding this high-visibility issue is a piece in the widely respected peer-reviewed journal Nature by U.S. Geological Survey\Woods Hole scientist Carolyn Ruppel. Quoting her from that report:
…some scientists raised the alarm that large quantities of methane (CH4) might be liberated by widespread destabilization of climate-sensitive gas hydrate deposits trapped in marine and permafrost-associated sediments (Bohannon 2008, Krey et al. 2009, Mascarelli 2009). Even if only a fraction of the liberated CH4 were to reach the atmosphere, the potency of CH4 as a greenhouse gas (GHG) and the persistence of its oxidative product (CO2) heightened concerns that gas hydrate dissociation could represent a slow tipping point (Archer et al. 2009) for Earth’s contemporary period of climate change.

Noting that methane is about 20 percent more potent a greenhouse gas than CO2 but oxidizes to CO2 after about a decade in the atmosphere, Ruppel writes that “The susceptibility of gas hydrates to warming climate depends on the duration of the warming event, their depth beneath the seafloor or tundra surface, and the amount of warming required to heat sediments to the point of dissociating gas hydrates.”
For those wanting to better understand the significance of methane in the whole global warming/climate change discussion, the Nature piece by Ruppel, chief of USGS’s Gas Hydrates Project, provides useful and practical information.
NITROUS OXIDE (N2O)
Nitrous oxide occurs naturally in Earth’s atmosphere as part of the nitrogen cycle. While it is the product of a wide variety of natural sources, human activities – agriculture, fossil fuel combustion, wastewater management and industrial processes – are increasing the atmospheric concentrations, the EPA says. In addition, nitrous oxide molecules in the atmosphere have long life spans – about 120 years before they are removed in a “sink” or destroyed as a result of chemical reactions. A pound of N2O gas has the equivalent warming effect of 300 times that of one pound of carbon dioxide.
Based on 2012 data, nitrous dioxide comprises about 6 percent of all U.S. emissions resulting from human activities. Globally, about two-fifths, 40 percent, of nitrous oxide emissions are attributable to human activities.
Agriculture, transportation and industry activities are major sources of nitrous oxide emissions, as indicated on this chart:

FLUORINATED GASES (HFCS, PFCS, SF6)
Fluorinated gases are emitted in smaller quantities than the other greenhouse gases, but what they lack in volume they can make up in potency and long lifespans in the atmosphere, ranging from 1-270 years for HFCs to 800-50,000 years for PFCs and about 3,200 years for SF6. Once emitted into the atmosphere, they disperse widely around the globe; they are removed from the atmosphere only by sunlight in the highest levels of the atmosphere. Being the most potent of the GHGs and having the longest lifespans, these gases often are described as the “high global warming potential (GWP) gases.”
Aluminum and semiconductor manufacturing processes are among the principal emitters of the fluorinated gases, as illustrated by this chart:

Carbon Sequestration as a Potential Climate Solution
Carbon sequestration helps slow or possibly reverse the effects of climate change. The USGS is exploring two major approaches to carbon dioxide removal and storage.
Geologic Carbon Sequestration
Geologic carbon sequestration involves storing carbon dioxide in stable rock formations. Technology captures carbon dioxide from industrial processes, like factories and power plants, and compresses the gas into a liquid. This liquid is then injected deep underground. Another technology is called carbon mineralization, which is the process by which carbon dioxide becomes a solid mineral, such as a carbonate. It is a chemical reaction that happens when certain rocks are exposed to carbon dioxide. The USGS is an international leader in identifying rock formations with high potential for carbon storage and is exploring the mechanisms and potential consequences of this process.

Biologic Carbon Sequestration
Biologic carbon sequestration takes advantage of nature’s ability to store carbon. Through photosynthesis, plants remove carbon dioxide from the atmosphere and use it as a building block to create new tissue. Some of the carbon remains preserved in soil, sediments, and wood. Ecosystems like forests and wetlands can absorb huge amounts of carbon dioxide from the atmosphere and store it for long time periods, from decades to thousands of years. The USGS helps managers and conservation agencies identify ecosystems that are particularly good at storing carbon and supports restoration and conservation of these areas. Much of this work currently focuses on carbon stored in coastal regions, known as “blue carbon.”

Global warming potential
The global warming potential (GWP) depends on both the efficiency of the molecule as a greenhouse gas and its atmospheric lifetime. GWP is measured relative to the same mass of CO2 and evaluated for a specific timescale. Thus, if a gas has a high (positive) radiative forcing but also a short lifetime, it will have a large GWP on a 20-year scale but a small one on a 100-year scale. Conversely, if a molecule has a longer atmospheric lifetime than CO2 its GWP will increase when the timescale is considered. Carbon dioxide is defined to have a GWP of 1 over all time periods.
Methane has an atmospheric lifetime of 12 ± 2 years. The 2021 IPCC report lists the GWP as 83 over a time scale of 20 years, 30 over 100 years and 10 over 500 years. A 2014 analysis, however, states that although methane’s initial impact is about 100 times greater than that of CO2, because of the shorter atmospheric lifetime, after six or seven decades, the impact of the two gases is about equal, and from then on methane’s relative role continues to decline. The decrease in GWP at longer times is because methane decomposes to water and CO2 through chemical reactions in the atmosphere.

Removal from the atmosphere
Natural processes
Greenhouse gases can be removed from the atmosphere by various processes, as a consequence of:
-
- a physical change (condensation and precipitation remove water vapor from the atmosphere).
-
- a chemical reaction within the atmosphere. For example, methane is oxidized by reaction with naturally occurring hydroxyl radical, OH· and degraded to CO2 and water vapor (CO2 from the oxidation of methane is not included in the methane Global warming potential). Other chemical reactions include solution and solid phase chemistry occurring in atmospheric aerosols.
-
- a physical exchange between the atmosphere and the other components of the planet. An example is the mixing of atmospheric gases into the oceans.
-
- a chemical change at the interface between the atmosphere and the other components of the planet. This is the case for CO2, which is reduced by photosynthesis of plants, and which, after dissolving in the oceans, reacts to form carbonic acid and bicarbonate and carbonate ions (see ocean acidification).
-
- a photochemical change. Halocarbons are dissociated by UV light releasing Cl· and F· as free radicals in the stratosphere with harmful effects on ozone (halocarbons are generally too stable to disappear by chemical reaction in the atmosphere).

Negative emissions
A number of technologies remove greenhouse gases emissions from the atmosphere. Most widely analyzed are those that remove carbon dioxide from the atmosphere, either to geologic formations such as bio-energy with carbon capture and storage and carbon dioxide air capture,or to the soil as in the case with biochar. The IPCC has pointed out that many long-term climate scenario models require large-scale human-made negative emissions to avoid serious climate change.
History of scientific research
This 1912 article succinctly describes how burning coal creates carbon dioxide that causes climate change.
In the late 19th century, scientists experimentally discovered that N2 and O2 do not absorb infrared radiation (called, at that time, “dark radiation”), while water (both as true vapor and condensed in the form of microscopic droplets suspended in clouds) and CO2 and other poly-atomic gaseous molecules do absorb infrared radiation. In the early 20th century, researchers realized that greenhouse gases in the atmosphere made Earth’s overall temperature higher than it would be without them. During the late 20th century, a scientific consensus evolved that increasing concentrations of greenhouse gases in the atmosphere cause a substantial rise in global temperatures and changes to other parts of the climate system, with consequences for the environment and for human health.

The future of greenhouse gas emissions and the prospects for limiting global warming
The devastating impacts of global warming, such as elevated global temperatures, melting ice caps in the polar regions, and increased cases of natural disasters such as earthquakes and Tsunamis, have peaked the calls for reduced carbon emission. This turn of events has necessitated hasty and responsive interventions to curb and suppress greenhouse gas emissions and foster adaptability to these climatic changes. According to the UN climatic change report of 2023, burning fossil fuels and using unsustainable energy sources have increased global temperatures to unprecedented levels threatening global sustainability. This report proposes a hasty and collaborative approach to deploy effective and equitable climate action plans to reduce the damage to nature and people and transform the unpredictable climatic patterns to secure a sustainable future for all living beings.
Conclusion
In conclusion, the greenhouse effect is a natural process that is essential for life on Earth. However, human activities, such as the burning of fossil fuels and deforestation, have significantly increased the concentration of greenhouse gases in the atmosphere, leading to an enhanced greenhouse effect and global warming.
The consequences of global warming are severe and can be seen in the form of rising sea levels, more frequent and intense heatwaves, droughts, and wildfires, among other things. These changes have significant impacts on human health, biodiversity, and economies around the world.
To mitigate the effects of global warming, it is essential to reduce greenhouse gas emissions through various measures such as transitioning to renewable energy sources, improving energy efficiency, promoting sustainable transportation, and adopting sustainable land use practices.
Moreover, we need to adopt adaptive measures to cope with the impacts of climate change that are already happening. These measures include improving infrastructure to withstand extreme weather events, implementing drought-resistant agriculture, and protecting vulnerable communities from the impacts of climate change.
Finally, it is crucial to raise awareness about the greenhouse effect and climate change, and engage people in adopting sustainable behaviors and lifestyles. Through collective action, we can work towards a sustainable future that ensures a livable planet for future generations.
Bibliography
https://en.wikipedia.org/wiki/Greenhouse_effect
https://education.nationalgeographic.org/resource/greenhouse-effect/
https://www.usgs.gov/science/science-explorer/climate/greenhouse-gases-and-carbon-storage










