Natural polymers are large molecules composed of repeating subunits found in nature. Learn about their properties, sources, and applications here.
[Click for ISC CHEMISTRY PRACTICAL]
We will provide some interesting Chemistry Research topics , this will help you to gain more knowledge about different spheres of chemistry. CLICK
INTRODUCTION
Polymer means large molecules whose structure is composed of multiple repeating units, from which originates a characteristic of high relative molecular mass and attendant properties. The small units by which a polymer is made up of is called monomer. So, a large number of monomers combine together to form a polymer.These polymers are formed either by the process of addition polymerization or condensation polymerization.
NEED OF HERBAL POLYMERS
- Biodegradable – Naturally occurring polymers produced by all living organisms. They show no adverse effects on the environment or human being.
- Biocompatible and non-toxic – Chemically, nearly all of these plant materials are carbohydrates in nature and composed of repeating monosaccharide units. Hence they are non-toxic.
- Economic – They are cheaper and their production cost is less than synthetic material.
- Safe and devoid of side effects – They are from a natural source and hence, safe and without side effects.
- Easy availability – In many countries, they are produced due to their application in many industries.
DISADVANTAGES OF HERBAL POLYMERS
- Microbial contamination – During production, they are exposed to external environment and hence, there are chances of microbial contamination.
- Batch to batch variation – Synthetic manufacturing is controlled procedure with fixed quantities of ingredients while production of natural polymers is dependent on environment and various physical factors.
- The uncontrolled rate of hydration—Due to differences in the collection of natural
- materials at different times, as well as differences in region, species, and climate conditions the percentage of chemical constituents present in a given material may vary.
- Slow Process – As the production rate is depends upon the environment and many other factors, it can’t be changed. So natural polymers have a slow rate of production.
- Heavy metal contamination – There are chances of Heavy metal contamination often associated with herbal excipients.
Polymers are of two types: naturally occurring and synthetic or man made.
NATURAL POLYMER
Natural polymers occur in nature and can be extracted. They are often water-based.Our body too is made up of many natural polymers like nucleic acids, proteins, etc. The Cellulose is another natural polymer which is a main structural component of the plants. Most of the natural polymers are formed from the condensation polymers and this formation from the monomers, water is obtained as a by-product.
Some Natural polymers also include DNA and RNA, these polymers are very much important in all the life processes of all the living organisms. This messenger RNA is the one that makes possible peptides, proteins, and enzymes in a living body. Enzymes inside the living organisms help the reactions to happen and the peptides makes up the structural components of hair, skin, and also the horns of a rhino. The other natural polymers are polysaccharides or called as sugar polymers and polypeptides such as keratin, silk, and the hair. Natural rubber is also a natural polymer which is made of hydrogen and carbon.
CLASSIFICATION OF NATURAL POLYMERS
- Plant origin – Cellulose, Hemicellulose, Glucomannan, Agar, Starch, Pectin, Inulin, Rosin, Guar gum, Locust bean Gum, Gum Acacia, Karaya gum, Gum Tragacanth, Aloe Vera gel.
- Animal origin – Chitin, Alginates, Carageenans, Psyllium, Xanthum gum.
Examples of Natural Polymers
There are about many examples of natural polymers which occur in nature. A brief description on some of them are listed below-
Proteins and Polypeptides::
Proteins are the basic type of natural polymers which constitutes in almost all the living organisms. Proteins are said to be most versatile in nature. They can also be as catalysts. Some proteins are called as enzymes. These enzymes are responsible for various chemical reactions occurring in our body and it happens about a million times faster even without these enzymes. One type of protein in our blood called as haemoglobin carries the oxygen from lungs to the cells of a human body.

A protein is usually a naturally occurring type of polyamide. This polymer consists of an amide group present in the backbone chain of human body.
Collagen:
Collagen is one of the natural polymers and is a protein. It makes up the connective tissue present in the skin of human beings. This Collagen-polymer is also a fiber that creates an elastic layer below the skin and thus helps in keeping it supple and smooth.
Latex:
Latex is known to be a kind of rubber, and rubber is a natural polymer. This latex occurs in both the forms either synthetic or natural. The natural form of latex is mainly collected from the rubber trees and it is also found in variety of plants which includes the milkweed. It can also be prepared artificially by the process of building up long chains of molecules of styrene.

Cellulose:
Cellulose is one of the most abundant organic compounds found on the Earth and moreover the purest form of natural cellulose is the cotton. The paper manufactured from the woods of trees and also the supporting materials in leaves and plants mainly comprise cellulose. Like the amylose, it is also a polymer which is made from the monomers of glucose.
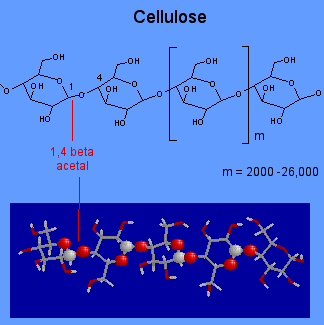
Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula. Cellulose is an organic polysaccharide with the formula (C6H10O5)n, consisting of a linear chain of several hundred to over ten thousand β(1→4) linked D-glucose units. The polysaccharides of the plant cell wall consist mainly of cellulose, hemicelluloses and pectin.11 Cellulose is an essential structural component of cell walls in higher plants and is the most abundant organic polymer on earth. Many parallel cellulose molecules form crystalline microfibrils that are mechanically strong and highly resistant to enzymatic attack. These are aligned with each other to provide structure to the cell wall. Cellulose is insoluble in water and indigestible by the human body.

Microcrystalline cellulose is mainly used in the pharmaceutical industry as a diluent/binder in tablets for both the granulation and direct compression processes.
Controlled release applications for cellulose derivatives include the formulation of membrane
controlled drug release systems or monolithic matrix systems. Film coating techniques for the manufacture of membrane controlled release systems include enteric coated dosage forms and the use of semi- permeable membranes in osmotic pump delivery systems.
Hydroxypropylmethylcellulose is a partly O- methylated and O-(2-hydroxypropylated) cellulose ether derivative that has been extensively investigated as an excipient in controlled release drug delivery systems due to its gel forming ability. In a study where two cellulose ethers; hydroxypropylmethylcellulose and carboxymethylcellulose were employed as polymeric carrier materials in matrix tablets for controlling the release of a soluble drug, diltiazem, it was found that each polymer on its own could sustain drug release over an extended period of time in these systems.
More importantly, a mixture of the two cellulose ethers in the matrix type tablets enabled zero order drug release kinetics at both pH 4.5 and 6.8. Hydroxypropylmethylcellulose monolithic matrix systems showed similar dissolution profiles as a commercial osmotic pump system for glipizide, a drug with low solubility. It was further found that the hydroxypropylmethylcellulose matrix systems have a stronger gel structure than those made of polyethylene oxide, which may provide superior in vivo performance in terms of matrix resistance to the destructive forces within the gastrointestinal tract.
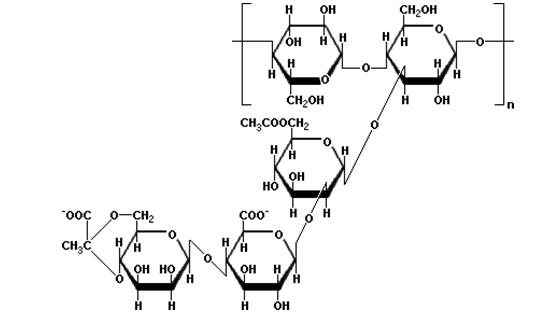
Hemicellulose
A hemicellulose is a heteropolymer (matrix polysaccharides), such as arabinoxylans, present along with cellulose in almost all plant cell walls. While cellulose is crystalline, strong, and resistant to hydrolysis, hemicellulose has a random, amorphous structure with little strength.
Unlike cellulose, hemicellulose (also a polysaccharide) consists of shorter chains – 500-3,000 sugar units. In addition, hemicellulose is a branched polymer, while cellulose is unbranched.
Hemicellulose polysaccharides consist of xyloglucans, xylans and mannans that can be extracted from the plant cell wall with a strong alkali. They have backbones made up of β-1,4-linked D- glycans. Xyloglucan has a similar backbone as cellulose, but contains xylose branches on 3 out of every 4 glucose monomers. The β-1,4-linked D- Xylan backbone of arabinoxylan contains arabinose branches polymer,while cellulose is unbranched.
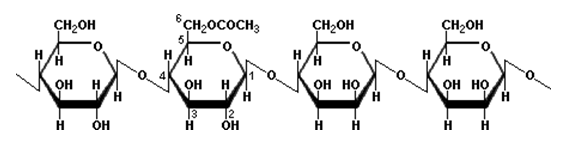
Glucomannan
Glucomannan is a hydrocolloidal polysaccharide of the mannan family consisting of β-1,4 linked D- mannose and D-glucose monomers (with acetyl side branches on some of the backbone units), but the mannose:glucose ratio may differ depending on the source. The acetyl groups contribute to its solubility and swelling capacity and assist in making it a soluble natural polysaccharide with the highest viscosity and water-holding capacity. It is very abundant in Nature and this polysaccharide is specifically derived from softwoods, roots, tubers and plant bulbs. The most commonly used type of Glucomannan is referred to as konjac Glucomannan, which is extracted from the tubers of Amorphophallus konjac and is a very promising polysaccharide for incorporation into drug delivery systems. Since konjac Glucomannan by itself forms very weak gels, it has been investigated as an effective exponent in controlled release drug delivery devices in combination with other polymers or by modifying its chemical structure.17, 18
It was shown that konjac Glucomannan gel systems were able to maintain the integrity and control the
release of theophylline and diltiazem for 8 hours. This was, however, dependent on the country of origin (i.e. Japan, Europe or America) due to differences in the degree of acetylation of the konjac Glucomannan.Matrix tablets prepared from konjac glucomannan alone showed the ability to sustain the release of cimetidine in the physiological environments of the stomach and small intestines but the presence of β-mannanase (colon) accelerated the drug release substantially. Mixtures of konjac Glucomannan and xanthan gum in matrix type tablets showed high potential to sustain and control the release of the drug due to stabilization of the gel phase of the tablets by a network of intermolecular hydrogen bonds between the two polymers to effectively retard drug diffusion. Konjac Glucomannan was used to form hydrophilic cylinders and particles for controlled release of DNA. Konjac Glucomannan cross-linked with trisodium trimetaphosphate formed hydrogel systems that could sustain hydrocortisone release dependent on cross- linking density and enzymatic degradation
Agar
Agar or agar-agar is the dried gelatinous substance obtained from Gelidium amansii (Gelidaceae) and several other species of red algae like, grailaria (Gracilariaceae) and Pterocladia (Gelidaceae).

Agar consists of a mixture of agarose and agaropectin. The predominant component, agarose, is a linear polymer, made up of the repeating monomeric unit of agarobiose. Agarobiose is a disaccharide made up of D-galactose and 3,6- anhydro-L-galactopyranose. Agaropectin is a heterogeneous mixture of smaller acidic molecules that gel poorly. Its great gelling power in an aqueous environment allows it to form gels which are more resistant (stronger) than those of any other gel- forming agent, assuming the use of equal concentrations. It can be used over a wide range of pH, from 5 to 8, and in some cases beyond these limits. It withstands thermal treatments very well, even above 100°C which allows good sterilization. A 1.5% aqueous solution gels between 32°C-43°C and does not melt below 85°C. This is a unique property of agar, compared to other gelling agents. Agar gives gels without flavour and does not need the additions of cations with strong flavours (potassium or calcium) it can be used without problems to gel food products with soft flavours. Its gel has an excellent reversibility allowing it to be repeatedly gelled and melted without losing any of the original properties. Agar is used as Suspending agent, emulsifying agent, gelling agent in suppositories, surgical lubricant, tablet disintegrants, medium for bacterial culture, laxative.
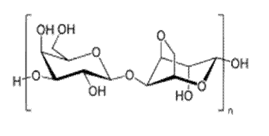
Starch:
Starch is the derivative of condensation polymerization and consists of glucose monomers, which further split into water molecules when combined chemically. Starch is also a member of basic food groups called the carbohydrates and it is found in the grains, cereal and potatoes. Starch is a polymer of monosaccharide glucose. The molecules of starch consist of 2 kinds of glucose polymers namely amylopectin and amylose which are the main component of starch in most of the plants.
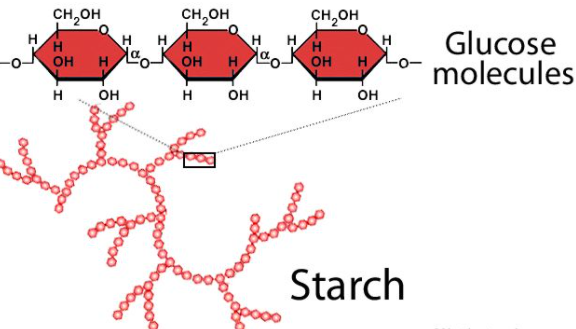
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store. It is the principal form of carbohydrate reserve in green plants and especially present in seeds and underground organs. Starch occurs in the form of granules (starch grains). A number of starches are recognized for
pharmaceutical use. These include maize (Zea mays), rice (Oryza sativa), wheat (Triticum aestivum), and potato (Solanum tuberosum).

It is comprised of two polymers, namely amylose (a non-branching helical polymer consisting of α-1, 4 linked D-glucose monomers) and amylopectin (a highly branched polymer consisting of both α-1,4 and α-1,6 linked D-glucose monomers).
Modified Starch
It was tested for general applicability of a new pregelatinized starch product in directly compressible controlled-release matrix systems.

It was prepared by enzymatic degradation of potato starch followed by precipitation (retrogradation), filtration and washing with ethanol. The advantages of the material include ease of tablet preparation, the potential of a constant release rate (zero-order) for an extended period of time and its ability to incorporate high percentages of drugs with different physicochemical properties. Release rates from retrograded pregelatinized starch tablets can be enhanced or decreased to the desired profile by different parameters like geometries of the tablet, compaction force and the incorporation of additional excipients.
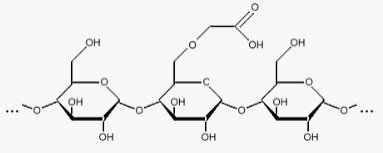
Native Starch
It may not be suitable in controlled release drug delivery systems due to substantial swelling and rapid enzymatic degradation resulting in too fast release of many drugs. This has led to the use of derivatives of starch that are more resistant to enzymatic degradation as well as crosslinking and formation of co-polymers. Starch acetate prepared by acetyl esterification has shown retarded enzymatic degradation with the potential to be used as a colon- targeted drug delivery carrier. High amylase carboxymethyl starch produced by spray drying showed a high loading capacity for the soluble drug, acetaminophen, in controlled release direct compressible matrix systems.

To deliver proteins or peptide drugs orally, microcapsules containing a protein and a proteinase inhibitor were prepared. Starch/bovine serum albumin mixed-walled microcapsules were prepared using interfacial cross-linking with terephthaloyl chloride. The microcapsules were loaded with native or amino-protected aprotinin by incorporating protease inhibitors in the aqueous phase during the cross-linking process. The protective effect of microcapsules with aprotinin for bovine serum albumin was revealed in vitro.
Pectin
Pectin is the purified carbohydrate product obtained by acid hydrolysis from the inner portion of the rind of citrus peels i.e. Citrus Simon or Citrus Aurantium, (Rutaceae). The main component of pectin is a linear polysaccharide composed of α-1,4-linked D- galacturonic acid residues interrupted by 1,2- linked L-rhamnose residues with a few hundred to about one thousand building blocks per molecule, corresponding to an average molecular weight of about 50,000 to about 1,80,000. The galacturonic acid polysaccharides are rich in neutral sugars such as rhamnose, arabinose, galactose, xylose and glucose. The composition of pectin can vary based on the botanical source, for example pectin from citrus contains less neutral sugars and has a smaller molecular size as compared to pectin obtained from apples.

Pectin has been investigated as an excipient in many different types of dosage forms such as film coating of colon-specific drug delivery systems when mixed with ethyl cellulose, microparticulate delivery systems for ophthalmic preparations and matrix type transdermal patches. It has high potential as a hydrophilic polymeric material for controlled release matrix drug delivery systems, but its aqueous solubility contributes to the premature and fast release of the drug from these matrices.
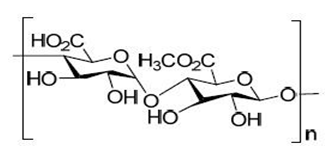
It was investigated that the suitability of amidated pectin as a matrix patch for transdermal chloroquine delivery in an effort to mask the bitter taste when orally administered. The results suggest that the pectin-chloroquine patch matrix preparation has potential applications for the transdermal delivery of chloroquine and perhaps in the management of malaria. Calcium pectinate nanoparticles to deliver insulin were prepared as a potential colonic delivery system by ionotropic gelation.
Micro particulate polymeric delivery systems have been suggested as a possible approach to improve the low bioavailability characteristics shown by standard ophthalmic vehicles (collyria). In this context pectin microspheres of piroxicam were prepared by the spray drying technique. In vivo tests in rabbits with dispersions of piroxicam-loaded microspheres also indicated a significant improvement of piroxicam bioavailability in the aqueous humour (2.5-fold) when compared with commercial piroxicam eye drops.
Depending on the type and structure of the pectin molecule, pectins can gel in various ways. Gelling can be induced by acid or cross-linking with calcium ion or by reaction with alginate. When a pectin solution is titrated with acid, the ionization of carboxylate groups on pectins is repressed causing pectin molecules to no longer repel each other over their entire chains. The pectins can thus associate over a portion of their chains to form acid-pectin gels. Gel forming systems have been investigated widely for sustained drug delivery. A mixture of xyloglucan with pectin resulted in an in situ gel forming system with sustained paracetamol drug delivery in rats.
In relation to cosmetics, using citronella as a model compound, pectin gel formulations were evaluated for controlled fragrance release by kinetic and static methods. These formulations showed a prolonged duration of fragrance release and limitation of fragrance adsorption to the receptor skin layers. The increase in pectin concentrations suppressed the fragrance release by a diffusion mechanism, thereby proving that pectin/calcium microparticles are promising materials for controlled fragrance release.
In relation to the food industry, folic acid incorporated microcapsules were prepared using alginate and combinations of alginate and pectin polymers so as to improve stability of folic acid. Folic acid stability was evaluated with reference to encapsulation efficiency, gelling and hardening of capsules, capsular retention during drying and storage. The blended alginate and pectin polymer matrix increased the folic acid encapsulation efficiency and reduced leakage from the capsules as
compared to those made with alginate alone, they showed higher folic acid retention after freeze drying and storage.
Inulin
It is a polysaccharide from the bulbs of Dehlia, Inula Helenium (Compositae), roots of Dendelion, Taraxacum officinale (Compositae). Burdock root, Saussurea lappa (Compositae) or chicory roots, Cichonium intybus (Compositae).
Inulin consists of a mixture of oligomers and polymers that belong to the group of gluco-fructans and occur in plants such as garlic, onion, artichoke and chicory. The inulin molecules contain from two to more than 60 fructose molecules linked by β-2,1- bonds. Inulin is resistant to digestion in the upper gastrointestinal tract, but is degraded by colonic microflora.
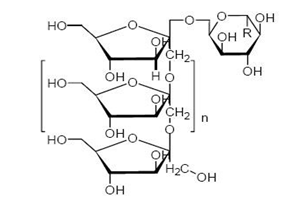
Inulin with a high degree of polymerization was used to prepare biodegradable colon-specific films in combination with Eudragit® RS that could withstand break down by the gastric and intestinal fluids. It was shown in another study where different Eudragits® were formulated into films with inulin that when a combination of Eudragit® RS and Eudragit® RL was mixed with inulin it exhibited better swelling and permeation properties in colonic medium rather than other gastrointestinal media. Methylated inulin hydrogels were developed as colon-specific drug delivery systems and investigated for water uptake and swelling. The hydrogels exhibited a relatively high rate of water uptake and anomalous dynamic swelling behaviour.
Inulin derivatised with methacrylic anhydride and succinic anhydride produced a pH sensitive hydrogel by UV irradiation that exhibited a reduced swelling and low chemical degradation in acidic medium, but it had a good swelling and degradation in simulated intestinal fluid in the presence of its specific enzyme, inulinase.
Rosin
Rosin, also called colophony or Greek pitch (Pix græca), is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene components. Rosin is a natural polymer with a low molecular weight of 400 Da obtained from the oleoresin of pine trees, with the principle sources being Pinus soxburghui, Pinus longifolium and Pinus toed a. Rosin is primarily composed of abietic and pimaric acids and has excellent film-forming properties. Rosin and its derivatives are biopolymers that are increasingly used for their pharmaceutical applications. In the pharmaceutical context it has been investigated for Microencapsulation, film-forming and coating properties, matrix materials in the tablets for sustained and controlled release.

Derivatives of rosin synthesized by a reaction with polyethylene glycol 200 and maleic anhydride proofed suitable for sustaining the drug release from matrix tablets and pellets. Polymerized rosin films containing hydrophobic plasticizers showed excellent potential as coating materials for the preparation of sustained release dosage forms. Different studies on the film forming and coating properties of rosin and the glycerol ester of maleic rosin demonstrated their potential to be used as coating materials for pharmaceutical products as well as in sustained- release drug delivery systems. It was shown that hydrocortisone loaded nanoparticles prepared from rosin could slowly release this model drug, which was dependent on the rosin content. This in vitro study demonstrated the potential of rosin for the production of effective nanoparticulate drug delivery systems.

Guar Gum
Guar gum is the powder of the endosperm of the seeds of Cyamopsis tetragonolobus Linn. (Leguminosae). Guar gum is also called guaran, clusterbean, Calcutta lucern, Gum cyamposis, Cyamopsis gum, Guarina, Glucotard and Guyarem. It is a galactomannans which is a linear polysaccharide consisting of (1→4)-diequatorially linked β-D- mannose monomers, some of which are linked to single sugar side-chains of α-D-galactose attached. Guar gum has a backbone composed of β-1,4 linked- D-mannopyranoses to which, on average, every alternate mannose an α-D-galactose is linked 1→6. The FDA has affirmed guar gum as generally safe. Guar gum has recently been highlighted as an inexpensive and flexible carrier for oral extended release drug delivery. Guar gum is particularly useful for colon delivery because it can be degraded by specific enzymes in this region of the gastrointestinal tract. The gum protects the drug while in the stomach and small intestine environment and delivers the drug to the colon where it undergoes assimilation by specific microorganisms or degraded by the enzymes excreted by these microorganisms. Guar gum on its own showed high potential to serve as a carrier for oral controlled release matrix systems. In addition, it was found that inclusion of excipients can be used as a tool to modulate drug release from these matrix systems.

Guar gum, in the form of three-layer matrix tablets, is a potential carrier in the design of oral controlled drug delivery systems for highly water-soluble drugs such as trimetazidine dihydrochloride. The same study was carried out by using metoprolol tartrate a model drug with high solubility. The results indicated that guar gum, in the form of three-layer matrix tablets, is a potential carrier in the design of oral controlled drug delivery systems for highly water- soluble drugs such as metoprolol tartrate.
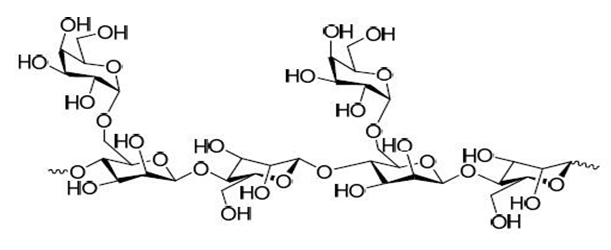
Another water soluble drug, diltiazem HCl has given controlled release comparable with marketed sustained release diltiazem HCl tablets (D-SR tablets), which are prepared in the form of matrix tablets with guar gum using the wet granulation technique.
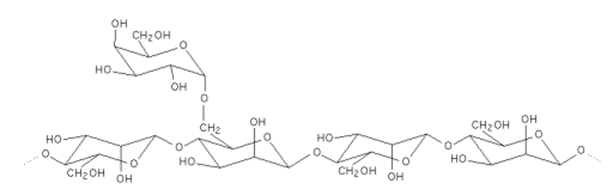
Locust Bean Gum
Locust bean gum also known as Carob bean gum is derived from the seeds of the leguminous plant Ceratonia siliqua Linn (Leguminosae). The brown pods or beans of the locust bean tree are processed by milling the endosperms to form locust bean gum and it is therefore not an extract of the native plant but flour. Locust bean gum consists mainly of a neutral galactomannan polymer made up of 1,4-linked D- mannopyranosyl units and every fourth or fifth chain unit is substituted on C6 with a D-galactopyranosyl unit. Locust bean gum is a neutral polymer and its viscosity and solubility are therefore little affected by pH changes within the range of 3-11.

Locust bean gum was used to produce matrix tablets with and without the cross-linker, glutaraldehyde that showed similar drug release profiles for different model drugs as guar gum and scleroglucan. In another study, sustained release of diclofenac sodium could be obtained for minimatrix systems made from locust bean gum. A commercially available tablet system (TIMERx®) developed by the Penwest Pharmaceuticals Company consisting of locust bean gum and xanthan gum showed both in vitro and in vivo controlled release potential.
Gum Arabic
Gum acacia or gum Arabic is the dried gummy exudation obtained from the stem and branches of Acacia Arabica wild, belonging to (Leguminosae). The gum has been recognized as an acidic polysaccharide containing D-galactose, L-arabinose, L-rhamnose, and D-glucuronic acid.
Acacia is mainly used in oral and topical pharmaceutical formulations as a suspending and emulsifying agent, often in combination with tragacanth. It is also used in the preparation of pastilles and lozenges and as a tablet binder.

Gum Arabic was successfully used as a matrix microencapsulating agent for the enzyme, endoglucanase, which proofed to give a slow release of the encapsulated enzyme and in addition increased its stability.
Gum Arabic was used as an osmotic suspending and expanding agent to prepare a monolithic osmotic tablet system. The optimum system delivered the water-insoluble drug, naproxen, at a rate of approximately zero order for up to 12 hours at a pH of 6.8. Sustained release of ferrous sulphate was achieved for 7 h by preparing gum Arabic pellets. The release was further sustained for more than 12 h by coating the pellets with polyvinyl acetate and ethylene vinyl acetate, respectively. An increase in the amount of gum Arabic in the pellets decreased the rate of release due to the gelling property of gum Arabic. The gel layer acts as a barrier and retards the rate of diffusion of FeSO4 through the pellet.
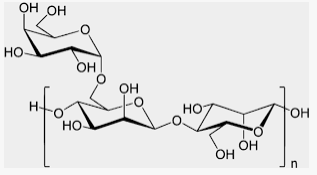
Karaya Gum
Karaya gum is obtained from Sterculia urens (Sterculiaceae) is a partially acetylated polymer of galactose, rhamnose, and glucuronic acid. Swellable hydrophilic natural gums like xanthan gum and karaya gum were used as release-controlling agents in producing directly compressed matrices. Caffeine and diclofenac sodium, which are having different solubilities in aqueous medium were selected for gum erosion, hydration and drug release studies using a dissolution apparatus (basket method) at two agitation speeds. It was concluded that drug release from xanthan and karaya gum matrices depended on agitation speed, solubility and proportion of the drug.

Both xanthan and karaya gums produced near the zero order drug release with the erosion mechanism playing a dominant role, especially in karaya gum matrices. 60 It was shown that mucoadhesive tablets prepared by karaya gum for buccal delivery, had superior adhesive properties as compared to guar gum and was able to provide zero-order drug release, but concentrations greater than 50% w/w may be required to provide suitable sustained release.
Tragacanth
This gum is obtained from the branches of Astragalus gummifer (Leguminosae). Tragacanth contains from 20% to 30% of a water-soluble fraction called tragacanthin (composed of tragacanthic acid and arabinogalactan). It also contains from 60% to 70% of a water-insoluble fraction called bassorin. Tragacanthic acid is composed of D-galacturonic acid, D-xylose, L-fructose, D-galactose, and other sugars. Tragacanthin is composed of uronic acid and arabinose and dissolves in water to form a viscous colloidal solution (sol), while bassorin swells to form a thick gel.
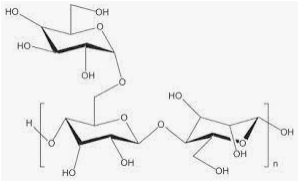
Tragacanth when used as the carrier in the formulation of 1- and 3-layer matrices produced satisfactory release prolongation either alone or in combination with other polymers.

As with other water-soluble gums, there is some preliminary evidence that concomitant ingestion of tragacanth with a high sugar load can moderate the blood sugar levels in patients with diabetes,64 although this effect has not been demonstrated consistently and requires much more detailed investigation. Although gum tragacanth swells to increase stool weight and decrease the GI transit time, it appears to have no effect on serum cholesterol, triglyceride or phospholipid levels after a 21-day supplementation period as do other soluble fibers.Tragacanth has been used since ancient times as an emulsifier, thickening agent, and suspending agent.
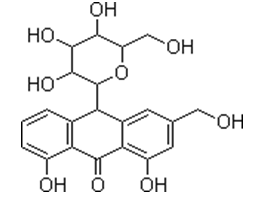
Aloe Gel
The inner part of the leaves of Aloe Vera (L.) Baum. f. (Aloe barbadensis Miller) consists of the parenchyma tissue that contains the mucilaginous gel.
After extraction of the A. Vera gel from the leaves and a filtration step, the acetone precipitate was directly compressed in matrix systems with diclofenac sodium as a model drug. The mucilage produced direct compressible matrix tablets that showed good swelling and sustained release of the model drug.

Many of the health benefits associated with Aloe Vera have been attributed to the polysaccharides contained in the gel of the leaves. These biological activities include promotion of wound healing, antifungal activity, hypoglycemic or antidiabetic effects antiinflammatory, anticancer, immunomodulatory and gastroprotective properties. These effects include the potential of whole leaf or inner fillet gel liquid preparations of A. Vera to enhance the intestinal absorption and bioavailability of co-administered compounds as well as enhancement of skin permeation. In addition, important pharmaceutical applications such as the use of the dried A. Vera gel powder as an excipient in sustained release pharmaceutical dosage forms.
Chitin
Chitin is the polysaccharide derivative containing amino and acetyl groups and are the most abundant organic constituent in the skeletal material of the invertebrates. It is found in mollusks, annelids, arthropods and also as a constituent of the mycelia and spores of many fungi. It may be regarded as a derivative of cellulose, in which the hydroxyl groups of the second carbon of each glucose unit have been replaced with acetamido (-NH(C=O)CH3) group.
The new polyelectrolyte complex gel beads based on Phosphorylated Chitosan (PCS) were developed for controlled release of ibuprofen in oral administration. The PCS gel beads were readily prepared from soluble phosphorylated chitosan by using an ionotropic gelation with counter polyanion, tripolyphosphate (TPP), at pH 4.0. Ibuprofen was highly loaded, around 90%, in the PCS gel beads. The release percents of ibuprofen from PCS gel beads were found to be increased as the pH of dissolution medium increased. Chitosan and their derivatives (N-trimethyl chitosan, mono-N-carboxymethyl chitosan) are effective and safe absorption enhancers to improve mucosal (nasal, peroral) delivery of hydrophylic macromolecules such as peptide and protein drugs and heparins. This absorption enhancing effect of chitosan is caused by the opening of the intercellular tight junctions, thereby favouring the paracellular transport of macromolecular drugs. Chitosan nano- and microparticles are also suitable for controlled drug release. Association of vaccines to some of these particulate systems has shown to enhance the antigen uptake by mucosal lymphoid tissues, thereby inducing strong systematic and mucosal immune responses against the antigens. The aspecific adjuvant activity of chitosans seems to be dependent on the degree of deacetylation and the type of formulation. From the studies reviewed it is concluded that chitosan and chitosan derivatives are promising polymeric excipients for mucosal drug and vaccine delivery.

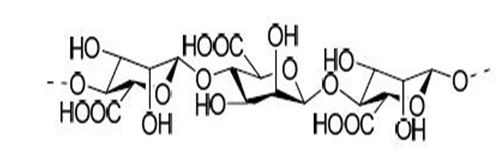
Alginates
Alginates or alginic acids is an anionic polysaccharide are linear, unbranched polysaccharides found in brown seaweed and marine algae such as Laminaria hyperborea, Ascophyllum nodosum and Macrocystis pyrifera.

Alginic acid can be converted into its salts, of which sodium alginate is the major form currently used. These polymers consist of two different monomers in varying proportions, namely β-D-mannuronic acid and α-L-guluronic acid linked in α- or β-1,4 glycosidic bonds as blocks of only β-D-mannuronic acid or α-L-guluronic acid in homopolymers or alternating the two in heteropolymeric blocks. Alginates have high molecular weights of 20 to 600 kDa.
Alginates have been used and investigated as stabilizers in emulsions, suspending agents, tablet binders and tablet disintegrants.
The in vivo delivery of anti-tuberculosis drugs were investigated in mice for alginate nanoparticles prepared by cation induced gelation. A single oral dose achieved therapeutic drug concentrations in the blood plasma for 7-11 days and in organs such as the lungs, liver and spleen for a total of 15 days. The drugs encapsulated in these nanoparticles resulted in significantly higher bioavailability compared to the
free drug. Furthermore, in M. Tuberculosis infected mice only three oral doses of the nanoparticles that were spaced 15 days apart resulted in complete bacterial clearance from specific organs, which is comparable to 45 conventional doses of the free drug.
Bioadhesive sodium alginate microspheres of metoprolol tartrate for intranasal systemic delivery were prepared to avoid the first-pass effect, as an alternative therapy for injection, and to obtain improved therapeutic efficacy in the treatment of hypertension and angina pectoris. The microspheres were prepared using emulsification-cross linking method. In vivo studies indicated significantly improved therapeutic efficacy of metoprolol from microspheres, with sustained and controlled inhibition of isoprenaline-induced tachycardia as compared with oral and nasal administration of drug solution.
In a comparative study, alginate formulation appeared to be better than the polylactide-co- glycolide (PLG) formulation in improving the bioavailability of two clinically important antifungal drugs-clotrimazole and econazole. The nanoparticles were prepared by the emulsion-solvent-evaporation technique in case of PLG and by the cation-induced controlled gelification in case of alginate.
Carrageenans
Carrageenan is sulphated polysaccharide extract of the seaweed called carrageen; or Irish moss, the red algae obtained from Chondrus Crispus (Rhodophyceae).Carrageenan extracted from seaweed is not assimilated by the human body and provides only bulk but no nutrition. There are three basic types of carrageenan – kappa (κ), iota (ι) and lambda (λ) respectively.The λ-type carrageenan results in viscous solutions but are non-gelling, while the κ- type carrageenan forms a brittle gel.

The ι-type carrageenan produces elastic gels. A study where the compaction ability of two κ- carrageenans (Gelcarin® GP-812 NF and GP-911NF) and one ι-carrageenan (Gelcarin® GP-379 NF) was investigated showed that these carrageenans are able to form strong compacts with a high elastic recovery. It was finally concluded from the results that the carrageenans investigated were suitable tableting excipients for the manufacturing of controlled-release tablets.
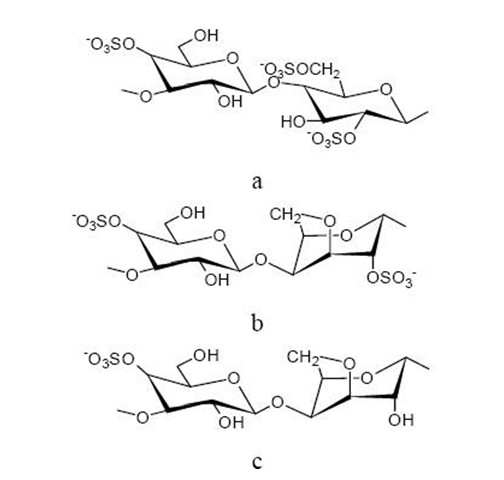
Hydrogel beads were prepared from a mixture of cross-linked κ-carrageenan with potassium and cross- linked alginate with calcium and they exhibited a smoother surface morphology than that of the one- polysaccharide network beads. The carrageenan parts of the hydrogen pronouncedly enhanced the thermostability of the polymeric network. These beads were introduced as novel carriers for controlled drug delivery systems.
Psyllium
Psyllium mucilage is obtained from the seed coat of Plantago ovata by milling the outer layer of the seeds. It has been evaluated for its tablet binding properties,but also to form hydrogels through radiation-induced cross-linking for controlled release of 5-fluorouracil as a model drug.

Psyllium and methacrylamide based hydrogels were prepared by using N,N’-methylenebisacrylamide as a cross-linker, which were then loaded with insulin.
These cross-linked hydrogels showed controlled release of the active ingredient by means of non- Fickian diffusion of the drug through polymer chain relaxation during swelling.81 Psyllium husk was used in combination with other excipients such as hydroxypropyl methylcellulose to prepare a novel sustained release, swellable and bioadhesive gastroretentive drug delivery systems for ofloxacin.
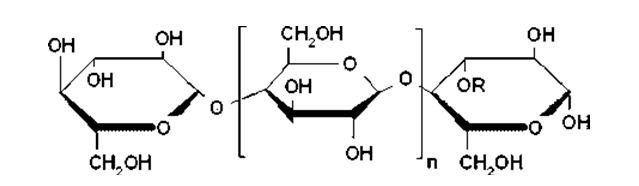
Xanthan Gum
Xanthan gum is a high molecular weight extracellular polysaccharide produced by the fermentation of the gram-negative bacterium Xanthomonas campestris. The primary structure of this naturally produced cellulose derivative contains a cellulosic backbone (β-D-glucose residues) and a trisaccharide side chain of β-D-mannose-β-D-glucuronicacid-α-D-mannose attached with alternate glucose residues of the main chain.
In one of the trials, xanthan gum showed a higher ability to retard the drug release than synthetic hydroxypropylmethylcellulose. Xanthan gum and hydroxypropylmethylcellulose were used as hydrophilic matrixing agents for preparing modified release tablets of diltiazem HCl. The amount of hydroxypropylmethylcellulose and xanthan gum exhibited significant effect on drug release from the tablets prepared by direct compression technique. It was concluded that by using a suitable blend of hydroxypropylmethylcellulose and xanthan gum desired modified drug release could be achieved.
By utilizing retention properties of xanthan gum and releasing properties of galactomannan, the desired release profile was achieved in delivering of theophylline. Hydrophilic galactomannan is obtained from the seeds of the Brazilian tree Mimosa scabrella (Leguminosae). The matrices made alone with xanthan gum (X) showed higher drug retention for all concentrations, compared with galactomannan (G) matrices that released the drug too fast. The matrices prepared by a combination of both gums were able to produce near zero-order drug release. The XG (conc. 8%) tablets provided the required release rate (about 90% at the end of 8 h), with zero-order release kinetics.

APPLICATIONS
Many of the applications of natural polymers lost out in the competitive world to synthetic polymers, in which properties could be tailored to needs. Petroleum-based products had properties independent of the material vagaries associated with crops, whose component properties can vary significantly with growin8 site, agronomic factors including weather, and other complicated considerations. Molecular weight, degree of branching, and chemical groups in side and main chain sites can be more readily modified and controlled for syna« pliers than for most natural materials. However, in some cases, chemical modifications of naMal polymers can lead to materials of particularly interesting properties; e.g., hydrolyzed starch/polyacrylonitrile graft copolymers with astounding water absorption and holding characteristics…the first superabsorbent (i4). In general, however, these problems caused natuml polymers to become comparatively unattractive at the commercial level. As a result, Morris emphasized that by the 1990’s two thirds of our clothes were derived from oil, plastics replaced paper containers, food dyes came from mineral oil and so on. These were all changes indicative of the shift of markets from natural to synthetic materials.
In recent years, the socio-economic situation has changed to make natural polymers once a8ain worth consideration for many applications. First of all, oil embargos and higher oil prices and concern over long-term availability of oil forced technologists and scientists to consider alternative, and especially renewable, sources of materials. Second, environmental considerations made the use of many natural materials very attractive because of their biodegradability, low toxicity and low disposal costs. Finally, properhes aside, the use of natural materials, such as starch, for fillers in composite materials was economically attractive because of the high price of synthetic polymer matrices and the low prices of such natural products as starch and starch granules. These low costs are related, of course, to the large agricultural surpluses of recent years and the ability of farmers to produce more. However, utilization of natural polymers only because they are cheap fillers hardly justifies their inclusion as “advanced materials.” To classify as advanced materials, there should be significant new and sophisticated technical features in their design, development, or processing. That is, there must be scientific or engineering factors, that go significantly beyond mere economic advantage, to warrant the designation “advanced.”
New Natural Polymers and Modeling. Computer based techniques have begun to be used to design and predict the properties of polymers, primarily of the synthetic type (2J). Some pioneering molecular modeling studies of plant proteins, starch and cellulose have been carried out recently . These have just scratched the surface of what is possible. The potential of emerging computer based methods to predict conformation, interactions and properties of natuml polymers, their chemical derivatives and blends is immense. This has become feasible for large molecules only recently as computer speed has increased and molecular dynamics simulations and new approximate methods have been developed.
Due to phenomenal advances in molecular biology, it may well become feasible in the near future to genetically manipulate plants (such as corn or soybeans) to produce pipe maalldefined molecular weight and branching and even introduce functional groups, such as acetyl, sulphate, amino and phosphate moieties. This may be easiest in the case of proteins, where the amino acid sequence follows directly from the sequence of bases in the plant’s DNA. For example, researchers are now modifying genes to improve the mechanical properties of animal proteins, such as elastin, silk and various adhesive polymers (i2). For other types of natural polymers, a« chemical structure is determined from DNA indirectly via the activity of a series of enzymes. Nevertheless, work on expression of bacterial genes for the production of poly(hydroxybutyrates) in plants is progressing and may eventually yield a polyester of very low cost . New genetic varieties of com containing starches of different branch frequency and length have recently been developed . The preparation of completely new polymers in plants may even be possible.
This points to the need for more understanding of the relationships between structure and function in natural polymers so we will know what kind of polymer we should make fora given application when the genetic/synthetic machinery is developed. Computer modeling may well become the “screening method” ofchoice to identify new polymers without having to go through the long and tedious process of synthesizing or “growing” every possibility. Also, it should not be forgotten that we currently raise only a dozen or so plant crops in large amounts and that the hundreds of thousands of other species of plants (not to menfion millions of species of microorganisms) represent an undiscovered source of new polymers and fiber. Exciting work for the botanical explorer!
Water Soluble and Swellnble Natural Polymers. Most natuml polymers are water swellable. Applications that take advantage of this property would seem to be prefered. Since many water soluble polymers are discharged directly into wastewater streams after use, the biodegradability of natural polymers would be an asset in preventing the build up or toxicity in fresh water sources. Water based polymer systems avoid the use of toxic organic solvents, the use of which is becoming more restricted by legislation. Also, the prices of synthetic, water soluble synthetic polymers are quite high, usually over Slflb. Thus, it is no surprise that starch and cellulose derivatives are widely used as water soluble adhesives, thickeners, binders and coatings (5, 12). Some emerging applications include polyaspartic acid and oxidized starches as metal ion sequestrants in detergents, mussel proteins as adhesives, cross-linked anionic starches as superabsorbents in drapers and modified agricultural residues as absorbents of organic wastes (43-48). More research is needed on basic stricture-property relationships of natural polymers to understand their behavior in aqueous systems.
NATURAL POLYMERS AND SUSTAINABILITY
In many ways, our current utilization of natural resources cannot be sustained. Most of our fuel for power and transportation comes from fossil fuels, such as oil which will be depleted in the future. Rising atmospheric carbon dioxide levels from combustion of fossil fuels are thought to be increasing global temperatures which, in turn, may cause droughts, crop losses, storm damage, etc. Soil for growing crops is being lost to erosion from wind and rain and the expansion of cities. Irrigation water, particularly
in the western U.S., is gradually being depleted as aquifer wells are pumped down. The number of species of living things in the world, estimated at >l0 million, are going extinct at a rate of about 0.3% per year (49). World population is presently growing at about 1.5% per year, while food production is increasing at only 0.5% per year [50). If these trends continue, world population will approximately double to 11 billion in 50 years.
How can natural polymers contribute solutions to these problems? The use of natural polymers as materials and as sources of fuel, such as ethanol, lessens our dependence on non-renewable petroleum. As pointed out by Orts and Glenn in this Symposium, certain natural polymer have the potential of acting as flocculating agents to control soil loss from erosion. Clearly, if the properties of a natural polymer, such as its strength, were improved, less would be needed for a specific application. New applications for agricultural wastes and byproducts of processing, as well as recycling of natural polymers, promise to make more efficient use of our natural resources. Also, alternative crops can produce more biomass per acre of land. Fiber from kenaf and hemp, rather than slow growing trees, can be utilized for a number of applications. This points to the need to conserve plant germplasm since solutions to future problems may lie in species as yet undiscovered.
CONCLUSION
Polymers play a vital role in the drug delivery. So, the selection of polymer plays an important role in drug manufacturing. But, while selecting polymers care has to be taken regarding its toxicity, drug compatibility and degradation pattern. By this review, we can say that natural polymers can be good substitute for the synthetic polymers and many of the side effects of the synthetic polymers can be overcome by using natural polymers.
Bibliography
- https://en.wikipedia.org/wiki/Polymer
- https://www.cmu.edu/gelfand/lgc-educational-media/polymers/natural-synthetic-polymers/index.html
- https://byjus.com/chemistry/natural-polymers/
- https://www.researchgate.net/publication/236217541_Natural_Polymers-_A_comprehensive_Review
- https://pubs.acs.org/doi/pdf/10.1021/bk-1999-0723.ch001
Acknowledgment
The success and final outcome of this project required a lot of guidance and assistance from many people and I am extremely privileged to have got this all along the completion of my project. All that I have done is only due to such supervision and assistance and I would not forget to thank them.
I respect and thank Mr./Ms. [NAME 1], for providing me an opportunity to do the project work in [VENUE] and giving us all support and guidance which made me complete the project duly. I am extremely thankful to [her/him] for providing such a nice support and guidance, although he had busy schedule managing the corporate affairs.
I owe my deep gratitude to our project guide [NAME 2], who took keen interest on our project work and guided us all along, till the completion of our project work by providing all the necessary information for developing a good system.
I would not forget to remember [NAME 3 AND NAME 4], of [COMPANY NAME] for their encouragement and more over for their timely support and guidance till the completion of our project work.
I heartily thank our internal project guide, [Name 5], [Position] , [Department] for her/his guidance and suggestions during this project work.
I am thankful to and fortunate enough to get constant encouragement, support and guidance from all Teaching staffs of [Department name] which helped us in successfully completing our project work. Also, I would like to extend our sincere esteems to all staff in laboratory for their timely support.
Some TIPS
1.Make the project more and more presentable.
2.Prefer colourful images like-
3. Paste downloaded images or draw images on the left side of the project copy.
4. Prefer good handwriting.
5. You may add statistical graphs, or sample in plastic pouches if required.
6. You may add the INDEX page before the INTRODUCTION page.
7. Colour the headings and subheadings.
8. Avoid using whitner .
9. Cover your project file with colorful chart paper and write your name, class, section, roll number, topic prominently.
10. PLEASE SUBMIT YOUR PROJECT ON TIME***










