Introductions:
In our daily life, we come across a number of chemical substances which make our life easy. Some of them are soap, nail polish, boot polish, varnish, nail polish remover, shampoo, perfumes, etc. From early morning to bedtime directly and indirectly we are constantly using chemical substances.
[Click for ISC CHEMISTRY PRACTICAL]
We will provide some interesting Chemistry Research topics , this will help you to gain more knowledge about different spheres of chemistry. CLICK
Soap
Soaps are the salts of fatty acids. Since they are salts of fatty acids, soaps have the general formula (RCO2−)nMn+ (Where R is an alkyl, M is a metal and n is the charge of the cation). The major classification of soaps is determined by the identity of Mn+. When M is Na or K, the soaps are called toilet soaps, used for handwashing. Many metal dications (Mg2+, Ca2+, and others) give metallic soap. When M is Li, the result is lithium soap (e.g., lithium stearate), which is used in high-performance greases.

Production of metallic soaps
Most metal soaps are prepared by neutralization of purified fatty acids:
RCO2H + CaO → (RCO2)2Ca + H2O
Production of toilet soaps
The production of toilet soaps usually entails the saponification of fats (triglycerides). Triglycerides are vegetable or animal oils and fats. An alkaline solution (often lye or sodium hydroxide) induces saponification whereby the triglyceride fats first hydrolyze into salts of fatty acids. Glycerol (glycerin) is liberated. The glycerin can remain in the soap product as a softening agent, although it is sometimes separated.
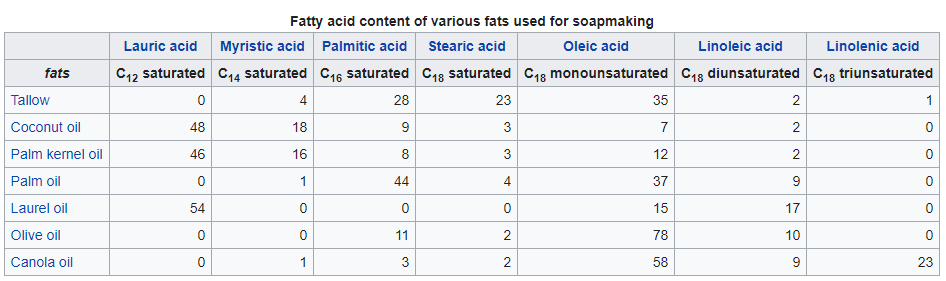
For making toilet soaps, triglycerides (oils and fats) are derived from coconut, olive, or palm oils, as well as tallow. Triglyceride is the chemical name for the triesters of fatty acids and glycerin. Tallow, i.e., rendered beef fat, is the most available triglyceride from animals. Each species offers quite different fatty acid content, resulting in soaps of distinct feel. The seed oils give softer but milder soaps. Soap made from pure olive oil, sometimes called Castile soap or Marseille soap, is reputed for its particular mildness. The term “Castile” is also sometimes applied to soaps from a mixture of oils, but a high percentage of olive oil.
Handmade soap from the cold process also differs from industrially made soap in that an excess of fat is used, beyond that needed to consume the alkali (in a cold-pour process, this excess fat is called “superfatting”), and the glycerol left in acts as a moisturizing agent. However, the glycerine also makes the soap softer. The addition of glycerol and processing of this soap produces glycerin soap. Superfatted soap is more skin-friendly than one without extra fat, although it can leave a “greasy” feel. Sometimes, an emollient is added, such as jojoba oil or shea butter. Sand or pumice may be added to produce a scouring soap. The scouring agents serve to remove dead cells from the skin surface being cleaned. This process is called exfoliation. The lye is dissolved in water.
To make antibacterial soap, compounds such as triclosan or triclocarban can be added. There is some concern that the use of antibacterial soaps and other products might encourage antibiotic resistance in microorganisms.
Nail Polish
Nail polish (also known as nail varnish or nail enamel) is a lacquer that can be applied to the human fingernail or toenails to decorate and protect the nail plates. The formula has been revised repeatedly to enhance its decorative effects and to suppress cracking or peeling. Nail polish consists of a mix of an organic polymer and several other components that give it its unique color and texture. Nowadays nail polishes come in all shades of colors and play a significant part in manicures or pedicures.

Preparation
- Nail polish consists of a film-forming polymer dissolved in a volatile organic solvent. Nitrocellulose that is dissolved in butyl acetate or ethyl acetate is common. This basic formulation is expanded to include the following:
- Plasticizers to yield non-brittle films. Dibutyl phthalate and camphor are typical plasticizers.
- Dyes and pigments. Representative compounds include chromium oxide greens, chromium hydroxide, ferric ferrocyanide, stannic oxide, titanium dioxide, iron oxide, carmine, ultramarine, and manganese violet.
- Opalescent pigments. The glittery/shimmer look in the color can be conferred by mica, bismuth oxychloride, natural pearls, and aluminum powder.
- Adhesive polymers ensure that the nitrocellulose adheres to the nail’s surface. One modifier used is tosylamide-formaldehyde resin.
- Thickening agents are added to maintain the sparkling particles in suspension while in the bottle. A typical thickener is stearalkonium hectorite. Thickening agents exhibit thixotropy, their solutions are viscous when still but free-flowing when agitated. This duality is convenient for easily applying the freshly shaken mixture to give a film that quickly rigidifies.
- Ultraviolet stabilizers resist color changes when the dry film is exposed to sunlight. A typical stabilizer is benzophenone-1.
Base coat
This type of nail polish is a clear, milky-colored, or opaque pink polish formula that is used specifically before applying nail polish to the nail. Its purpose is to strengthen nails, restore moisture to the nail, and help polish adhere to the nail. It prevents staining and extends the lifespan of the manicure. Some base coats are marketed as “ridge fillers”, and can create a smooth surface, de-emphasizing the ridges that can appear on unbuffed nails. Some base coats, called “peel off base coats”, allow the user to peel off their nail polish without using a remover.
Top coat
This type of nail polish is a clear colored polish formula that is used specifically after applying nail polish to the nail. It forms a hardened barrier for the nail that can prevent chipping, scratching, and peeling. Many topcoats are marketed as “quick-drying.” Topcoats can help the underlying colored polish dry quickly as well. It gives the polish a more finished and desired look and may help to keep the polish on for longer. Manganese violet is a typical pigment in nail polish.
Gel
Gel polish is a long-lasting variety of nail polish made up of a type of methacrylate polymer. It is painted on the nail similar to traditional nail polish but does not dry. Instead, it is cured under an ultraviolet lamp or ultraviolet LED. While regular nail polish formulas typically last two to seven days without chipping, gel polish can last as long as two weeks with proper application and home care. Gel polish can be more difficult to remove than regular nail polish. It is usually removed by soaking the nails in pure acetone (the solvent used in most nail polish removers) for five to fifteen minutes, depending on the formula.
Matte
Matte polish is like regular polish but has a purposely dull finish rather than a shine. It can be purchased as a regular base coat in ranges of different colors. Matte nail polish can also be found in a topcoat. Matte topcoat is most useful for painting over any dry base color, giving it a different appearance. The matte topcoat polish will dull the shine from a regular base coat polish. Matte polish has become very popular over the years, particularly since it can be used in nail art applications, where designs can be created on the nail using the contrast of both shiny and matte surfaces.
There are 17 principal nail polish finishes:
- Shimmer
- Micro-shimmer
- Micro-glitter
- Glitter
- Frost
- Lustre
- Creme
- Iridescent
- Opalescent
- Matte
- Duochrome
- Jelly or translucent
- Magnetic
- Crackled
- Glass-flecked
- Holographic
- Prismatic micro-glitter or shimmer
Nail Polish Remover
Nail polish remover is an organic solvent that may also include oils, scents, and coloring. Nail polish remover packages may include individual felt pads soaked in remover, a bottle of liquid remover used with a cotton ball or cotton pad, or a container filled with foam into which one inserts a finger and twists it until the polish comes off. Choosing a type of remover is determined by the user’s preference, and often the price or quality of the remover.

The most common remover is acetone. Repeated use can cause the skin around the nails to become dry or cracked. Acetone can also remove artificial nails made of acrylic or cured gel.
An alternative nail polish remover is ethyl acetate, which often also contains isopropyl alcohol. Ethyl acetate is usually the original solvent for nail polish itself.
Acetonitrile has been used as a nail polish remover, but it is more toxic and potentially more carcinogenic than the aforementioned options. It has been banned in the European Economic Area for use in cosmetics since 17 March 2000.
Boot Polish
Shoe polish (or boot polish) is a waxy paste, cream, or liquid used to polish, shine, and waterproof leather shoes or boots to extend the footwear’s life and restore, maintain and improve their appearance. Shoe polishes are distinguished by their textures, which range from liquids to hard waxes. Solvent, waxes, and colorants comprise most shoe polishes
Preparation:
The process for producing shoe polish is straightforward and the required equipment is relatively easy to acquire. The cost of establishing shoe polish manufacturing facilities has been estimated at around $600,000 (as of 2005).

Shoe polish is manufactured in large, thermostated, stirred reactors. Steps are taken to ensure that volatile solvents do not evaporate. Typically, low-melting paraffin wax is melted, followed by the higher melting waxes, and finally the colorant-stearate mixture. The molten mass is added to a warm solvent before being dispensed. Wax-based shoe polish is traditionally packaged in flat, round, 60-gram (2-ounce) tins, usually with an easy-open facility. The traditional flat, round tins have since become synonymous with shoe polishes. When dried due to solvent loss or other reasons, the hardened wax pulls away from the walls of the container giving what is known as a “rattler”.
Wax-based shoe polish
Nigrosin is a common dye in black shoe polish.
Waxes, organic solvents, and dyes compose this type of polish. Waxes are 20–40% of the material. Natural waxes include carnauba and montan as well as synthetic waxes. The composition determines the hardness and polishing properties after the solvent has evaporated. Solvents are selected to match the waxes. About 70% of shoe polish is solvent. A variety of solvents are used including naphtha. Turpentine, although more expensive, is favored for its “shoe polish odor”. Dyes make up the final 2–3% of the polish. A traditional dye is a nigrosine, but other dyes (including azo dyes) and pigments are used for oxblood, cordovan, and brown polishes.
Owing to its high content of volatile solvents, wax-based shoe polish hardens after application, while retaining its gloss. Poorly blended polishes are known to suffer from blooming, evidenced by the appearance of a white coating of stearin on the polish surface.
Cream-Emulsion shoe polish
These polishes may have a gelatinous consistency. They are composed of the usual three components waxes, liquid vehicles, and dyes. Unlike wax-based shoe polishes, cream-emulsions contain water and/or oil plus a solvent (either naphtha, turpentine, or Stoddard Solution), so the liquid content is high. Emulsifiers and surfactants are required. These include ammonia, morpholine, and various ethoxylated surfactants such as polysorbate 80. The waxes are often some mixture of carnauba wax, beeswax, montan wax and its oxidized derivatives, and paraffin waxes.
Liquid shoe polish
Liquid shoe polish is sold in a squeezable plastic bottle, with a small sponge applicator at the end. To decrease its viscosity, bottled polish usually has a very low wax content. Liquid shoe polish is a complex mixture. Polyethylene wax emulsion is a major component. Various polymers, typically acrylates, are the next major component, conferring gloss and holding the dyes in suspension. Resins and casein are selected to ensure adhesion to the leather. Fatty phosphate esters, emulsifiers, and glycols are also used. Pigments include titanium dioxide for whites and iron oxides for browns. Although liquid polish can put a fast shine on shoes, many experts warn against its long-term use because it can cause the leather to dry out and crack.[4]
Varnish
The varnish is a clear transparent hard protective finish or film. Varnish has little or no color and has no added pigment as opposed to painting or wood stain which contains pigment. However, some varnish products are marketed as a combined stain and varnish. The varnish is primarily used in wood finishing applications where the natural tones and grains in the wood are intended to be visible. It is applied over wood stains as a final step to achieve a film for gloss and protection. Varnish finishes are usually glossy but may be designed to produce satin or semi-gloss sheens by the addition of “flatting” agents.

Preparation
The varnish is traditionally a combination of drying oil, a resin, and a thinner or solvent. However, different types of varnish have different components. After being applied, the film-forming substances in varnishes either harden directly, as soon as the solvent has fully evaporated, or harden after evaporation of the solvent through curing processes, primarily chemical reaction between oils and oxygen from the air (autoxidation) and chemical reactions between components of the varnish.
Resin varnishes “dry” by evaporation of the solvent and hardens almost immediately upon drying. Acrylic and waterborne varnishes “dry” upon evaporation of the water but will experience an extended curing period. Oil, polyurethane, and epoxy varnishes remain liquid even after evaporation of the solvent but quickly begin to cure, undergoing successive stages from liquid or syrupy, to tacky or sticky, to dry gummy, to “dry to the touch”, to hard.
Environmental factors such as heat and humidity play a very large role in the drying and curing times of varnishes. In the classic varnish, the cure rate depends on the type of oil used and, to some extent, on the ratio of oil to resin. The drying and curing time of all varnishes may be sped up by exposure to an energy source such as sunlight, ultraviolet light, or heat.
There are many different types of drying oils, including linseed oil, tung oil, and walnut oil. These contain high levels of polyunsaturated fatty acids. Drying oils cure through an exothermic reaction between the polyunsaturated portion of the oil and oxygen from the air. Originally, the term “varnish” referred to finishes that were made entirely of resin dissolved in suitable solvents, either ethanol (alcohol) or turpentine.
Many different kinds of resins may be used to create a varnish. Natural resins used for varnish include amber, kauri gum, dammar, copal, rosin (colophony or pine resin), sandarac, balsam, elemi, mastic, and shellac. Varnish may also be created from synthetic resins such as acrylic, alkyd, or polyurethane. A varnish formula might not contain any added resins at all since drying oils can produce a varnish effect by themselves.
Originally, turpentine or alcohol was used to dissolve the resin and thin the drying oils. The invention of petroleum distillates has led to turpentine substitutes such as white spirit, paint thinner, and mineral spirit. Modern synthetic varnishes may be formulated with water instead of hydrocarbon solvents.
Various epoxies have been formulated as varnishes or floor finishes whereby two components are mixed directly before application. Often, the two parts are of equal volume and are referred to as “part A” and “part B”. True polyurethanes are two-part systems. All two-part epoxies have a “pot-life” or “working time” during which the epoxy can be used. Usually the pot-life is a matter of a few hours but is also highly temperature dependent. Both water-borne and solvent-based epoxies are used.
Spar varnish (also called marine varnish) was originally intended for use on ship or boat spars, to protect the timber from the effects of sea and weather. Spars bend under a load of their sails. The primary requirements were water resistance and also elasticity, so as to remain adhering as the spars flexed. Elasticity was a pre-condition for weatherproofing too, as a finish that cracked would then allow water through, even if the remaining film was impermeable. Appearance and gloss were of relatively low value. Modified tung oil and phenolic resins are often used.
Shampoo
The shampoo is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in bar form, like a bar of soap. The shampoo is used by applying it to wet hair, massaging the product into the scalp, and then rinsing it out. Some users may follow a shampooing with the use of hair conditioner.
Preparation
The shampoo is generally made by combining a surfactant, most often sodium lauryl sulfate or sodium laureth sulfate, with a co-surfactant, most often cocamidopropyl betaine in water to form a thick, viscous liquid. Other essential ingredients include salt (sodium chloride), which is used to adjust the viscosity, a preservative, and fragrance. Other ingredients are generally included in shampoo formulations to maximize the following qualities:
- pleasing foam
- ease of rinsing
- minimal skin and eye irritation
- thick or creamy feeling
- pleasant fragrance
- low toxicity
- good biodegradability
- slight acidity (pH less than 7)
- no damage to hair
- repair of damage already done to the hair
Many shampoos are pearlescent. This effect is achieved by the addition of tiny flakes of suitable materials, e.g. glycol distearate, chemically derived from stearic acid, which may have either animal or vegetable origins. Glycol distearate is a wax. Many shampoos also include silicone to provide conditioning benefits.

Commonly used ingredients[edit]
- Ammonium chloride
- Ammonium lauryl sulfate
- Glycol
- Sodium laureth sulfate is derived from coconut oils and is used to soften water and create a lather. There was some concern over this particular ingredient circa 1998 as evidence suggested it might be a carcinogen, and this has yet to be disproved, as many sources still describe it as irritating to the hair and scalp.
- Sodium lauryl sulfate
- Sodium lauroamphoacetate is naturally derived from coconut oils and is used as a cleanser and counter-irritant. This is the ingredient that makes the product tear-free.
- Polysorbate 20 (abbreviated as PEG(20)) is a mild glycol-based surfactant that is used to solubilize fragrance oils and essential oils, meaning it causes the liquid to spread across and penetrate the surface of a solid (i.e. hair).
- Polysorbate 80 (abbreviated as PEG(80)) is a glycol used to emulsify (or disperse) oils in water (so the oils do not float on top like Italian salad dressing).
- PEG-150 distearate is a simple thickener.
- Citric acid is produced biochemically and is used as an antioxidant to preserve the oils in the product. While it is a severe eye-irritant, the sodium lauroamphoacetate counteracts that property. Citric acid is used to adjust the pH down to approximately 5.5. It is a fairly weak acid which makes the adjustment easier. Shampoos usually are at pH 5.5 because at slightly acidic pH, the scales on a hair follicle lie flat, making the hair feel smooth and look shiny. It also has a small amount of preservative action. Citric acid, as opposed to any other acid, will prevent bacterial growth.
- Quaternium-15 is used as a bacterial and fungicidal preservative.
- Polyquaternium-10 has nothing to do with the chemical quaternium-15; it acts as the conditioning ingredient, providing moisture and fullness to the hair.
- Di-PPG-2 myreth-10 adipate is a water-dispersible emollient that forms clear solutions with surfactant systems
- Methylisothiazolinone, or MIT, is a powerful biocide and preservative.
Perfumes
Perfume is a mixture of fragrant essential oils or aroma compounds, fixatives, and solvents used to give the human body, animals, food, objects, and living-spaces an agreeable scent. It is usually in liquid form and used to give a pleasant scent to a person’s body.

Preparation
Although there is no single “correct” technique for the formulation of a perfume, there are general guidelines as to how a perfume can be constructed from a concept. Although many ingredients do not contribute to the smell of a perfume, many perfumes include colorants and anti-oxidants to improve the marketability and shelf life of the perfume, respectively.
Perfume oils usually contain tens to hundreds of ingredients and these are typically organized in a perfume for the specific role they will play. These ingredients can be roughly grouped into four groups:
- Primary scents (Heart): Can consist of one or a few main ingredients for a certain concept, such as “rose”. Alternatively, multiple ingredients can be used together to create an “abstract” primary scent that does not bear a resemblance to a natural ingredient. For instance, jasmine and rose scents are commonly blends for abstract floral fragrances. Cola flavourant is a good example of an abstract primary scent.
- Modifiers: These ingredients alter the primary scent to give the perfume a certain desired character: for instance, fruit esters may be included in a floral primary to create a fruity floral; calone and citrus scents can be added to create a “fresher” floral. The cherry scent in cherry cola can be considered a modifier.
- Blenders: A large group of ingredients that smooth out the transitions of a perfume between different “layers” or bases. These themselves can be used as a major component of the primary scent. Common blending ingredients include linalool and hydroxycitronellal.
- Fixatives: Used to support the primary scent by bolstering it. Many resins, wood scents, and amber bases are used as fixatives.
The top, middle, and base notes of a fragrance may have separate primary scents and supporting ingredients. The perfume’s fragrance oils are then blended with ethyl alcohol and water, aged in tanks for several weeks and filtered through processing equipment to, respectively, allow the perfume ingredients in the mixture to stabilize and to remove any sediment and particles before the solution can be filled into the perfume bottles.
Bibliography
- https://en.wikipedia.org/wiki/Soap
- https://en.wikipedia.org/wiki/Nail_polish#cite_note-7
- https://en.wikipedia.org/wiki/Shoe_polish#:~:text=A%20vigorous%20rubbing%20action%20to,drop%20of%20water%20or%20spit.
- https://en.wikipedia.org/wiki/Varnish
- https://en.wikipedia.org/wiki/Shampoo
- https://en.wikipedia.org/wiki/Perfume
- https://acknowledgementsample.com/2013/03/24/acknowledgement-sample-for-school-project/
Acknowledgment:
The success and final outcome of this project required a lot of guidance and assistance from many people and I am extremely privileged to have got this all along the completion of my project. All that I have done is only due to such supervision and assistance and I would not forget to thank them.
I respect and thank Mr./Ms. [NAME 1], for providing me an opportunity to do the project work in [VENUE] and giving us all support and guidance which made me complete the project duly. I am extremely thankful to [her/him] for providing such a nice support and guidance, although he had busy schedule managing the corporate affairs.
I owe my deep gratitude to our project guide [NAME 2], who took keen interest on our project work and guided us all along, till the completion of our project work by providing all the necessary information for developing a good system.
I would not forget to remember [NAME 3 AND NAME 4], of [COMPANY NAME] for their encouragement and more over for their timely support and guidance till the completion of our project work.
I heartily thank our internal project guide, [Name 5], [Position] , [Department] for her/his guidance and suggestions during this project work.
I am thankful to and fortunate enough to get constant encouragement, support and guidance from all Teaching staffs of [Department name] which helped us in successfully completing our project work. Also, I would like to extend our sincere esteems to all staff in laboratory for their timely support.
Some TIPS
1.Make the project more and more presentable.
2.Prefer colourful images like-
3. Paste downloaded images or draw images on the left side of the project copy.
4. Prefer good handwriting.
5. You may add statistical graphs, or sample in plastic pouches if required.
6. You may add the INDEX page before the INTRODUCTION page.
7. Colour the headings and subheadings.
8. Avoid using whitner .
9. Cover your project file with colorful chart paper and write your name, class, section, roll number, topic prominently.
10. PLEASE SUBMIT YOUR PROJECT ON TIME***










