Hello dear students, in this post I will try to explain how you will write your chemistry project. It carries 10 marks. So don’t take it lightly. First, decide the topic on which you will make the project. You have to opt for any one of the 15 topics suggested by the council.
We will provide some interesting Chemistry Research topics , this will help you to gain more knowledge about different spheres of chemistry. CLICK
[Click For ISC CHEMISTRY PRACTICAL]
(ISC CHEMISTRY SEMISTER-I ANSWER KEY- CLICK)
1. Amino acids (Click)
2. Nucleic acid (Click)
4. Vitamins and hormones (Click)
5. Simple idea of chemical evolution (Click)
6.Natural polymers (Click)
7.Types of Dyes (Click)
8.Chemicals in medicines (Click)
9. Preparation of soaps, nail polish, boot polish, varnish, nail polish remover, shampoo, and perfumes. (Click)
10.Chemicals and chemical processes in forensic studies. (Click)
11. Insecticides, pesticides and chemical fertilizers (Click)
12.Ancient Indian medicines and medicinal plants.(Click)
13.Organic Chemistry in nutrition, Food science, and biotechnology.(Click)
14. Effect of green House Gases. (Click)
15. How plastics have changed the world, both socially and economically.(Click)
The following points should be present in a project-
1.Introduction
2. Contents [within 20 to 25 pages]
3. Bibliography
4. Acknowledgment
Types of Dyes
Introduction:
A dye is a colored compound, normally used in solution, which is capable of being fixed to a fabric. The dye must be ‘fast’ or chemically stable so that the color will not wash with soap and water, fade on exposure to sunlight, etc. Dyeing is normally done in a special solution containing dyes and particular chemical material. After dyeing, dye molecules have an uncut Chemical bond with fiber molecules. The temperature and time controlling are two key factors in dyeing. The dyes were obtained from animal, vegetable, or mineral origin with no or very little processing. By far the greatest source of dyes has been from the plant kingdom, notably roots, berries, bark, leaves, and wood, but only a few have ever been used on a commercial scale.
*** ALWAYS START FROM A FRESH PAGE FOR ANY SUB-SECTION***
A) History of Dye:
textile dyeing dates back to the Neolithic period. Throughout history, people have dyed their textiles using common, locally available materials. Scarce dyestuffs that produced brilliant and permanent colors such as the natural invertebrate dyes Tyrian purple and crimson kermes were highly prized luxury items in the ancient and medieval world. Plant-based dyes such as woad, indigo, saffron, and madder were important trade goods in the economies of Asia and Europe. Across Asia and Africa, patterned fabrics were produced using resist dyeing techniques to control the absorption of color in piece-dyed cloth. Dyes from the New World such as cochineal and logwood were brought to Europe by the Spanish treasure fleets, and the dyestuffs of Europe were carried by colonists to America.
yed flax fibers have been found in the Republic of Georgia in a prehistoric cave dated to 36,000 BP. Archaeological evidence shows that, particularly in India and Phoenicia, dyeing has been widely carried out for over 5,000 years. Early dyes were obtained from animal, vegetable, or mineral sources, with no to very little processing. By far the greatest source of dyes has been from the plant kingdom, notably roots, berries, bark, leaves, and wood, only a few of which are used on a commercial scale.
Ancient hieroglyphs describe the extraction and application of natural dyes. Countless attempts have been made to extract dyes from brightly colored plants and flowers, yet only a dozen or so natural dyes found widespread use. Undoubtedly most attempts failed because most natural dyes are not highly stable and occur as components of complex mixtures, the successful separation of which would be unlikely by the crude methods employed in ancient times. Nevertheless, studies of these dyes in the 1800s provided a base for the development of synthetic dyes, which dominated the market by 1900.
he first synthetic dye, mauve, was discovered serendipitously by William Henry Perkin in 1856. The discovery of mauveine started a surge in synthetic dyes and in organic chemistry in general. Other aniline dyes followed, such as fuchsine, safranine, and induline. Many thousands of synthetic dyes have since been prepared. The discovery of mauve also led to developments within immunology and chemotherapy. In 1891 Paul Ehrlich discovered that certain cells or organisms took up certain dyes selectively. He then reasoned that a sufficiently large dose could be injected to kill pathogenic microorganisms if the dye did not affect other cells. Erlich went on to use a compound to target syphilis, the first time a chemical was used in order to selectively kill bacteria in the body, he also used methylene blue to target the plasmodium responsible for malaria.
B) Principles of color:
Dyes possess color because they –
1) absorb light in the visible spectrum (400–700 nm)
2) have at least one chromophore (color-bearing group)
3) have a conjugated system, i.e. a structure with alternating double and single bonds.
4) exhibit resonance of electrons, which is a stabilizing force in organic compounds.
When any one of these features is lacking from the molecular structure the color is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (color helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for color, their presence can shift the color of a colorant and they are most often used to influence dye solubility.
Regarding the requirement of a chromophore generating color in organic compounds, it is important to note that the chromophore must be part of a conjugated system. Placement of an azo group between methyl groups produces a colorless compound, while a yellow-orange color is obtained when the azo group is placed between aromatic rings.
Having an extended conjugated system causes a significant bathochromic shift, i.e. to a darker color.In addition to influencing solubility, auxochromes are essential ring substituents in providing target colors.
Effects of substituent groups within an azo-dye system
- Adding groups of increasing electron-donating ability to the azobenzene structure has a bathochromic effect .
- Electron-donating (NH2) and electron-accepting (NO2) groups placed in conjugation provide a bathochromic effect. In this regard, nitro groups are especially beneficial, contributing to their prevalence in disperse dye structures.
- Increasing the number of electron-attracting groups conjugated with the electron-donor has a bathochromic effect.
- The electron-donating effects of an amino group are enhanced by adding alkyl groups to the N-atom.
C)The major differences between dyes and pigments :
| Dyes | Pigments | |
| Solubility | Soluble in many liquids | Insoluble in water and most of the solvents |
| Number | Available in a large number | Comparatively lesser in number |
| Light fastness | Lower dyes are very much vulnerable. Lights destroy colored objects by breaking open electronic bonding within the molecule | Traditionally pigments have been found to be more light fast than dyes |
| Product resistance | Lower as compared to pigments | Very high |
| Size | Dye molecules are comparatively smaller; it’s like comparing a football (pigment) to say a head of a pin (dye) | Pigment particles are about 1-2 microns in size. It means that the particles can be seen under a magnifying glass |
| Bonding | Dye molecules have electrostatic charges that serve as a method for attaching the dye to the concrete | Pigment requires the help if a binder for gluing. As it is an inert substance which si merely suspended in a carrier/binder |
| Imparting colors | Dyes can impart color by selective absorption of the dyes | Pigments impart colors by either scattering of light or by selective absorption |
| Combustible Properties | Combustible | Non-combustible |
| Chemical Composition | Organic compounds | Normally inorganic compounds, often involving heavy toxic metals |
| Longevity | Do not last as long as pigments | Last longer than dyes |
D) Natural and Synthetic Dyes:
Natural dyes are simply dyed substances extracted from natural sources. Although the main source of dyes for early times, they have largely been replaced by synthetic dyes, which are usually more reliable, cheaper and can be supplied more readily. Natural dyes still in use include hematoxylin, carmine, orcein.
Colouring materials have been used for many thousands of years by man. Leather, cloth, food, pottery and housing have all been modified in this way. Some of our most common dyes are still derived from natural sources. These are termed natural dyes. The Colour Index uses this as a classification and naming system.
Each dye is named according to the pattern:
Natural + base colour + number
Natural dyes are often negatively charged. Positively charged natural dyes do exist but are not common. In other words, the colored part of the molecule is usually the anion. Although the molecular charge is often shown on a specific atom in structural formulae, it is the whole molecule that is charged. Many, but by no means all, natural dyes require the use of a mordant.
Synthetic dye:
Dyes derived from an organic or inorganic compound are known as synthetic dyes. Examples of this class of dyes are Direct, Acid, Basic, Reactive, Mordant, Metal complex, Vat, Sulfur, Disperse dye, etc. Synthetic dyes quickly replaced the traditional natural dyes.
They cost less, they offered a vast range of new colors, and they imparted better properties to the dyed materials dyes are now classified according to how they are used in the dyeing process.
E) Types of Dyes:
Acid dye:
Acid dyes are water-soluble anionic dyes, containing one or more sulfonic acid substituents or other acidic groups. An example of the class is Acid Yellow 36.
 |
| Acid yello36 |
Acid dyes are water-soluble anionic dyes that are applied to fibers such as silk, wool, nylon and modified acrylic fibers using neutral to acid dye baths. Acid dyes are not substantive to cellulosic fibers. Most synthetic food colors fall in this category. The dyeing process is reversible and may be described as follows:
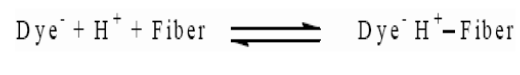
Basic or Cationic Dye:
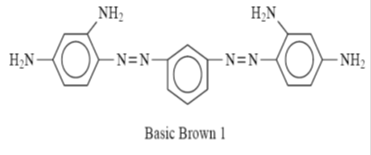
This group was the first of the synthetic dyes to be taken out of coal-tar derivatives. As textile dyes, they have been largely replaced by later developments. They are still used in discharge printing, and for preparing leather, paper, wood, and straw. More recently they have been successfully used with some readymade fibers, especially the acrylics. Basic dyes were originally used to color wool, silk, linen, hemp, etc., without the use of a mordant, or using agent. With a mordant like tannic acid, they were used on cotton and rayon. Basic dyes give brilliant colors with exceptional fastness to acrylic fibers. They can be used on basic dyeable variants of nylon and polyester.
Basic Brown 1 is an example of a cationic dye that is readily protonated under the pH 2 to 5 conditions of dyeing.
 |
| Basic Brown 1 |
Direct Dye:
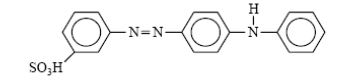
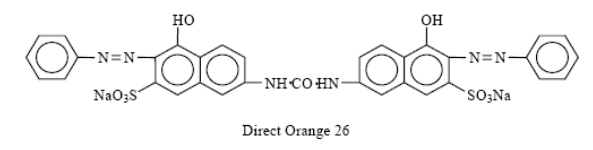
These are the dyes which can be applied directly to the fabrics from an aqueous solution. These are most useful for fabrics which can form hydrogen bonds with the Dyeing of Fabrics. The direct dyes mainly the basic dyes and were widely hailed because they made it unnecessary to use a mordant or binder in dyeing cotton. The colors are not as brilliant as those in the basic dyes but they have better fastness to light and washing, and such fastness can be measurably improved by after treatments (diazotized and developed.) Direct dyes can be used on cotton, linen, rayon, wool, silk and nylon. These dyes usually have azo linkage –N=N- and high molecular weight. They are water soluble because of sulfonic acid groups.
Direct orange 26 is a typical direct dye.
 |
| Direct orange 26 |
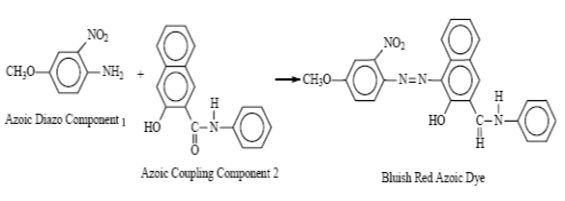
Azoic Dye:
Azo dyes contain at least one azo group (-N=N-) attached to one or often two aromatic rings. These dyes are used primarily for bright red shades in dyeing and printing since most other classes of fast dyes are lacking in good red dyes. Azoic dyes, called Naphthols in the industry, are actually manufactured in the fabric by applying one half of the dye. The other half is then put on and they combine to form the finished color. Unless they are carefully applied and well washed, they have poor fastness to rubbing or crocking.
The production of bluish red azoic dye from the following two components is an example.
Nitro Dye:
Nitro dyes are polynitro derivatives of phenols containing at least one nitro group ortho or para to the hydroxyl group.It is used to dye wool.It Consist of two or more aromatic rings (benzene, naphthalene).
Example:
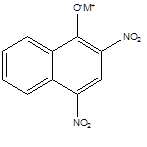 |
| Maritus yellow |
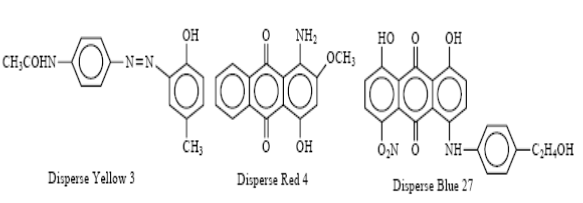
Disperse Dye:
Disperse dyes were originally developed for dyeing secondary cellulose acetate fibers. These dyes are relatively insoluble in water and are prepared for dyeing by being ground into relatively fine powder in the presence of dispersing agents. In the dye bath, a suspension of the dye particle dispersion produces a very dilute solution of the dyes, which are then absorbed by the fibers. This dye class is used to dye polyester, nylon, acetate and triacetate fibers.
Disperse yellow 3, Disperse Red 4, and Disperse Blue 27 are good examples of disperse dyes.
Example:
Vat Dye:
The vat dyes are insoluble complex polycyclic molecules based on the quinone structure (ketoforms). The term vat comes from the old indigo method dyeing in a vat: indigo had to be reduced to light form. Vat dyes are made from indigo, anthraquinone and carbazole. They are successfully used on cotton, linen, rayon, wool, silk, and sometimes nylon. Vat dyes are also used in the continuous piece of dyeing process sometimes called the pigment application process. The dyeings produced in this way have high wash and light fastness.
An example of a vat dye is Vat Blue 4 (Indanthrene).
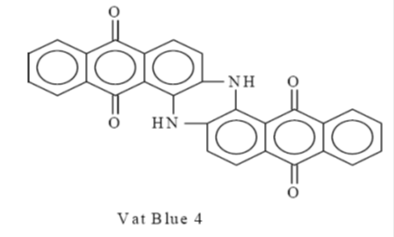 |
| Vat Blue 4 |
Mordant Dye:
These Dyeing of Fabrics do not dye the fabric directly but require a binding agent known as mordant. The mordant acts as a binding agent between the fibre and the dye. Some dyes combine with metal salts (mordanting) to form insoluble colored complexes (lakes). These materials are usually used for the dyeing of cotton, wool or other protein fiber. The metallic precipitate is formed in the fiber producing very fast colors highly resistant to both light and washing.
Example:

Reactive Dye:
These dyes react with the cellulosic fiber to form a covalent bond. This produces dyed fiber with extremely high wash fastness properties. These are the dyeing of fabrics which contain a reactive group which combines directly with the hydroxyl or the amino group of the fibre. Because of the chemical reaction the colour is fast and has a very long life. Cotton, wool or silk can be dyed with this type of dyeing of Fabrics.
Example: This type is the Reactive Blue 5 dye shown below,
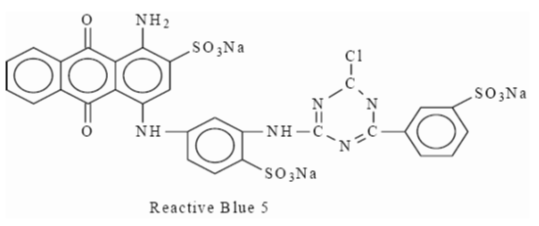 |
| Reactive Blue 5 |
Solvent Dye:
These dyes are water-insoluble but soluble in alcohols, chlorinated hydrocarbons, or liquid ammonia. These colours are applied by dissolving in the target, which is invariably a lipid or non-polar solvent. The Colour Index uses this as a classification and naming system. Each dye is named according to the pattern: – solvent + base colour + number They are used for coloring synthetics, plastics, gasoline, oils and waxes.
Example:
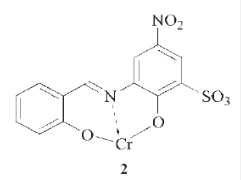 |
| Solvent yellow32 |
Sulfur Dye:
The sulphur dyes provide very deep shades, which have excellent resistance to washing but poor resistance to sunlight. They will dye cotton, linen, and rayon, but not brightly. A problem with sulphur dyes especially the black colors is that they make the fabric tender, or weaken its structure, so that it breaks easily. Sulfur dyes are applied to cotton from an alkaline reducing bath with sodium sulfide as the reducing agent. They are low cost and have good fastness to light, washings and acids.
Example:
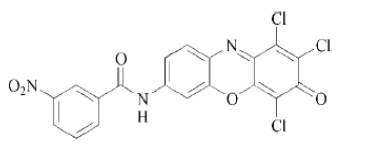 |
| Sulfur red 7 |
F) Properties of dyes:
- These dyes are economical dyes and are generally used to produce dark shades such as dark greens , dark blues and blacks.
- These dyes have good leveling and color fastness properties.
- The interaction between fiber and dye is established through very strong ionic bonds , which are formed between the anionic groups of the colorant and ammonium cations on the fiber. Chromium or the metal ion acts as bridge between the dye and fiber , which gives rise to a very strong linkage , resulting into excellent fastness properties.
Some dye application:
| Name of Dyes | Application |
| Acid dye | Man made fiber (Nylon),Natural fiber (Silk, Wool) |
| Direct Dye | Man made fiber (Viscose),Natural fiber (Cotton) |
| Vat dye | Man made fiber (Viscose),Natural fiber (Cotton, Silk, Wool) |
| Disperse dye | Nylon, Polyester, Acrylic, Tri-acetate, Di-acetate |
| Basic dye | Jute, Acrylic |
| Reactive dye | Cotton, Wool, Silk, Viscose, Nylon |
| Sulfur dye | Cotton, Viscose |
| Mordant dye | Cotton, Wool, Silk |
| Pigment | Cotton, Man made fiber |
| Mineral | Cotton, Wool, Silk |
| Azoic dye | Cotton, Viscose |
| Aniline Black | Cotton |
| Rapid and Rapidson dye | Cotton |
| Onium dye | Cotton, Jute |
Examples of few important natural dyes which are widely used in the dyeing of textile materials described below.
1. Jack fruits (Artocarpus heterophyllus Lam)
It is a very popular fruit of south India and other parts of India. The wood of the tree is cut into small chips and crushed into dust powder and then subsequently boiled in water to extract the dye. After mordanting treatment of dyed fabrics, yellow to brown shades are obtained. The cotton and jute fabrics are dyed by this dye. It belongs to the family of Moraceae. The dye consists of morin as a coloring molecule.
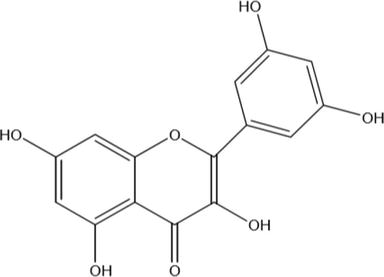
2 Turmeric (Curcuma longa)
The dye is obtained from the root of the plant. The turmeric root is dried, crushed in powder form and boiled with water to extract the dye. It can be used in the dyeing of cotton, wool, and silk. Proper mordanting treatment improves colour fastness to wash. The brilliant yellow shade is obtained after dyeing with turmeric natural dye. Turmeric is a rich source of phenolic compounds known as curcuminoids. The colouring ingredients in turmeric are called curcumin. Curcumin is diarylheptanoid existing in keto-enol form. Turmeric is a member of Curcuma botanical group .
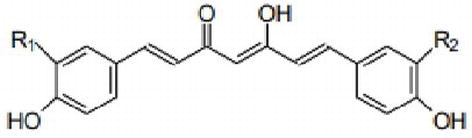
3. Onion (Allium cepa)
The papery skin of onion is the main source of the dye. Onion skin is boiled to extract the colour and subsequently can be dyed with or without mordanting the fabric. The resulting colour is from orange to brown. It contains colouring pigments called pelargonidin (5,5,7,4 tetrahydroxy antocyanidol). The amount of colouring pigment present varies from 2.0 to 2.25% .
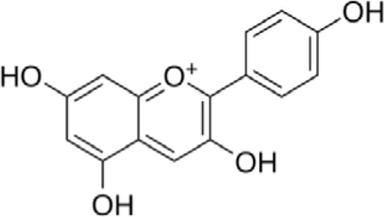
4 Hina (Lawsonia inermis L)
It is the leaf of the plant that is traditionally used in making the coloured design on the hands of women. The leaf of the plant is dried, crushed and subsequently boiled with water to extract the dye from leaf. The mordanted fabric gives colour from brown to mustard yellow. This is the dispersed dye type colour; hence, polyester and nylon can be dyed by hina. However, it stains wool and silk giving a lighter brown colour. Hina is commonly known as lawsone. The chief constituent of hina leaves is hennotannic acid; it is a red orange pigment. Chemically hennotannic acid is 2-hydroxy-1,4-naphthoquinone. The colouring molecules have strong substantivity for protein fibre .
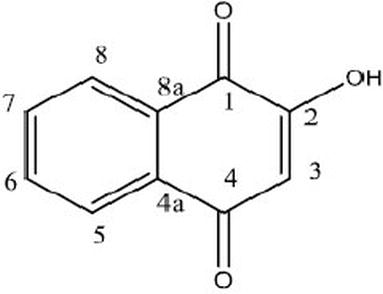
5 Indigo (Indigofera tinctoria)
It is the seed of the plant. The full matured plant has 0.4% colour on weight of the plant. The plants are steeped in the water until the fermentation start. When the hydrolysis of glucoside is completed, the liquor is separated from the plant debris. The extract is aerated which converts indoxyl to indigotin which separates out as a precipitate. The shade of natural indigo is difficult to reproduce exactly. The variety of blue shade on cotton can be obtained by the application of natural Indigo. It is kind of vat dye and hence need reductive vatting with liquid jiggery and citric acid or dithionate.
The precursor to indigo is indican which is a colourless water-soluble compound. Indican hydrolyzes in water and releases β-D-glucose and indoxyl. The oxidation of indoxyl resulted in indigotin. The average yield of indican from an indigo plant is 0.2–0.8%. Indigo is also present in molluscs. The molluscs contain mixture of indigo and 6,6′-dibromo indigo (red), which together produce a colour known as Tyrian purple. During dyeing due to air exposure, dibromo indigo is converted into indigo blue, and the mixture produces royal blue colour.
6 Madder or manjistha or Rubia (Rubia tinctorum)
The dye is obtained from the root of the plant. The root is scrubbed, dried in sunlight and finally boiled in the water to extract the dye in solution. The dye has red colour. The cotton, silk and wool fibre can be dyed with madder at a temperature of 100°C for time period of 60 min, and subsequently dye solution is cooled. Bright red shade is produced on wool and silk and red violet colour on cotton. This is a mordantable type of acid dye having phenolic (-OH) groups. The colouring matter in madder is alizarin of the antharaquinone group. The root of the plant contains several polyphenolic compounds, which are 1,3-dihydroxyanthraquinone, 1,4-dihydroxyanthraquinone, 1,2,4-trihydroxyanthraquinone and 1,2-dihydroxyanthraquinone .
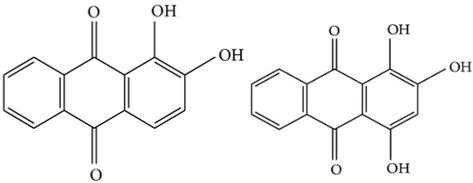
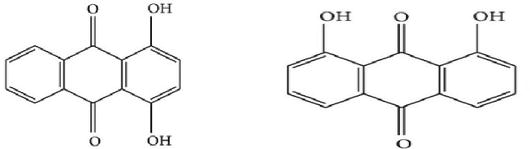
7 Tea waste (Camellia sinensis)
India is one of the biggest consumer of tea. The left over waste of tea is collectable in large quantity. The extract of tea waste can be used as a natural dye in combination with different mordants, which can produce yellowish brown to brown shade. This is a mordantable dye. Flavonoids, flavonols and phenolic acids are the main colouring components in waste of the tea. Polyphenols, which are mostly flavonols, are known as catechins with epicatechin and its derivatives.
8 Safflower (Carthamus tinctorius)
The safflower petals are soaked in distilled water and subsequently boiled with water for more than 2 h, and it is repeated two times. The solution is filtered and the filtrate is vacuum dried. The obtained powder is having strength of 20–30%. In dyeing it produces cherry red to yellowish red shade. Safflower contains natural pigment called carthamine. The biosynthesis of carthamine takes place by chalcone (2,4,6,4-tetrahydroxy chalcone) with two glucose molecules and that resulted in the formation of safflor A and safflor B.
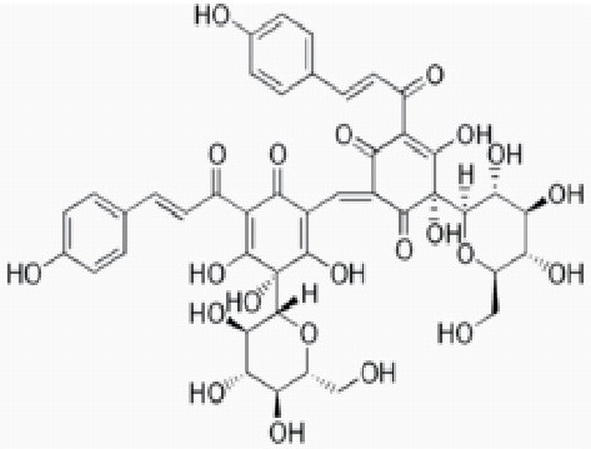
9 Sappan wood (Caesalpinia sappan)
Aqueous extraction is used to extract the dye from sappan wood. Alkali extraction can also be used. It produces bright red colour. It produces an orange colour in combination with turmeric and maroon shade with catechu. The sappan wood tree is found in India, Malaysia and the Philippines. The colouring pigment is similar to logwood. The same dye is also present in Brazil wood.
Bibliography:
- https://en.wikipedia.org/wiki/Dye#:~:text=A%20dye%20is%20a%20coloured,to%20the%20material%20they%20colour.
- https://www.britannica.com/technology/dye
- https://textilelearner.blogspot.com/2011/12/dye-classification-of-dye-according-to.html
- https://www.ncbi.nlm.nih.gov/books/NBK385442/
- https://textilelearner.blogspot.com/2015/01/different-types-of-dyes-with-chemical.html
- https://www.acs.org/content/acs/en/careers/college-to-career/chemistry-careers/dyes-pigments-ink.html
- https://blog.duraamen.com/differences-between-dyes-and-pigments/#:~:text=The%20major%20difference%20between%20dyes,to%20which%20they%20are%20applied.
- https://www.intechopen.com/online-first/fundamentals-of-natural-dyes-and-its-application-on-textile-substrates
- https://acknowledgementsample.com/2013/03/24/acknowledgement-sample-for-school-project/
Acknowledgment:
The success and final outcome of this project required a lot of guidance and assistance from many people and I am extremely privileged to have got this all along the completion of my project. All that I have done is only due to such supervision and assistance and I would not forget to thank them.
I respect and thank Mr./Ms. [NAME 1], for providing me an opportunity to do the project work in [VENUE] and giving us all support and guidance which made me complete the project duly. I am extremely thankful to [her/him] for providing such a nice support and guidance, although he had busy schedule managing the corporate affairs.
I owe my deep gratitude to our project guide [NAME 2], who took keen interest on our project work and guided us all along, till the completion of our project work by providing all the necessary information for developing a good system.
I would not forget to remember [NAME 3 AND NAME 4], of [COMPANY NAME] for their encouragement and more over for their timely support and guidance till the completion of our project work.
I heartily thank our internal project guide, [Name 5], [Position] , [Department] for her/his guidance and suggestions during this project work.
I am thankful to and fortunate enough to get constant encouragement, support and guidance from all Teaching staffs of [Department name] which helped us in successfully completing our project work. Also, I would like to extend our sincere esteems to all staff in laboratory for their timely support.
Some TIPS
1.Make the project more and more presentable.
2.Prefer colourful images like-
3. Paste downloaded images or draw images on the left side of the project copy.
4. Prefer good handwriting.
5. You may add statistical graphs, or sample in plastic pouches if required.
6. You may add the INDEX page before the INTRODUCTION page.
7. Colour the headings and subheadings.
8. Avoid using whitner .
9. Cover your project file with colorful chart paper and write your name, class, section, roll number, topic prominently.
10. PLEASE SUBMIT YOUR PROJECT ON TIME***










