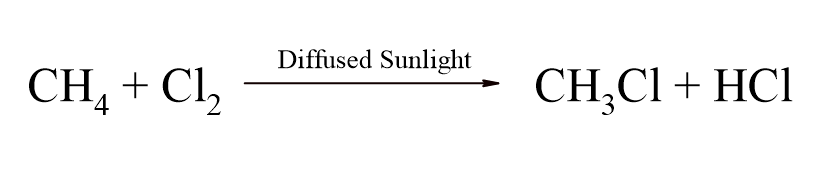
The synthesis of methyl chloride (chloromethane) from methane is a common reaction in organic chemistry. It is a free-radical substitution reaction that typically occurs when a mixture of methane (CH4) and chlorine gas (Cl2) is exposed to ultraviolet (UV) light or high temperatures. The reaction proceeds in three main steps:
- Initiation: The UV light provides the energy to break the chlorine molecule (Cl2) into two highly reactive chlorine free radicals (Cl⋅). This is an example of homolytic fission. Cl2→2Cl⋅
- Propagation: The chlorine radical reacts with a methane molecule, abstracting a hydrogen atom to form a methyl radical (CH3⋅) and hydrogen chloride (HCl). The methyl radical then reacts with another chlorine molecule to form methyl chloride (CH3Cl) and another chlorine radical. This new radical can continue the chain reaction. CH4+Cl⋅→CH3⋅+HCl CH3⋅+Cl2→CH3Cl+Cl⋅
- Termination: The reaction stops when two free radicals combine to form a stable molecule. This can be two chlorine radicals, a chlorine radical and a methyl radical, or two methyl radicals. Cl⋅+Cl⋅→Cl2 CH3⋅+Cl⋅→CH3Cl CH3⋅+CH3⋅→CH3CH3 (ethane)
A major challenge with this method is that the reaction doesn’t easily stop at methyl chloride. The newly formed methyl chloride can continue to react with chlorine radicals, leading to a mixture of products including dichloromethane (CH2Cl2), trichloromethane (CHCl3), and tetrachloromethane (CCl4). To maximize the yield of methyl chloride, a large excess of methane is typically used in the reaction.














