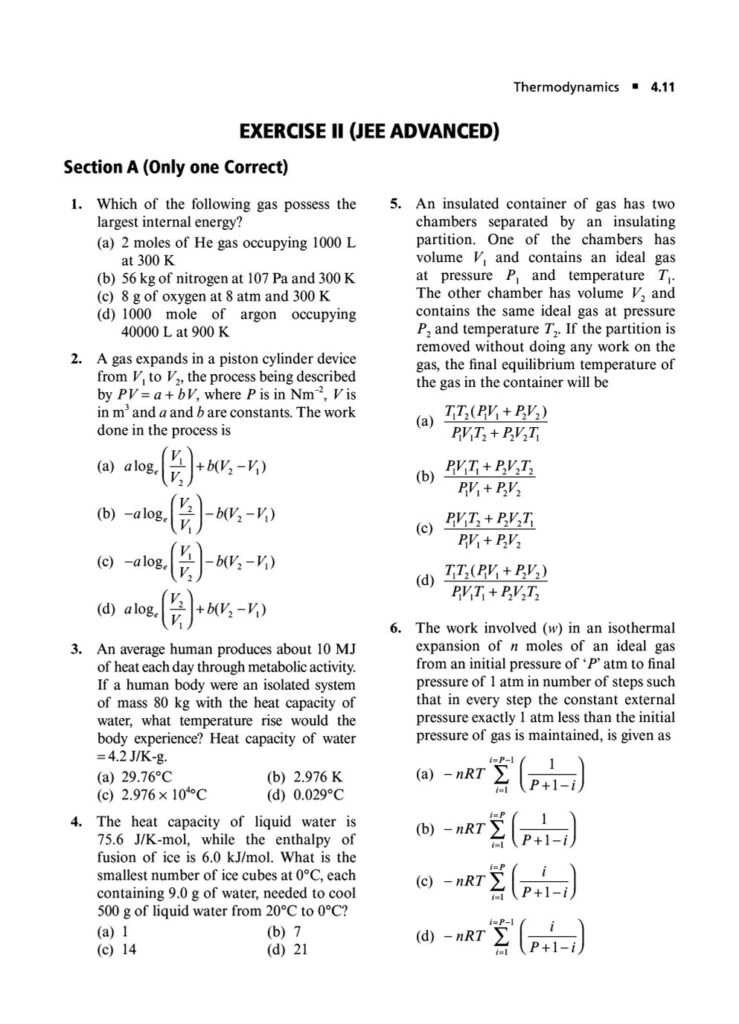
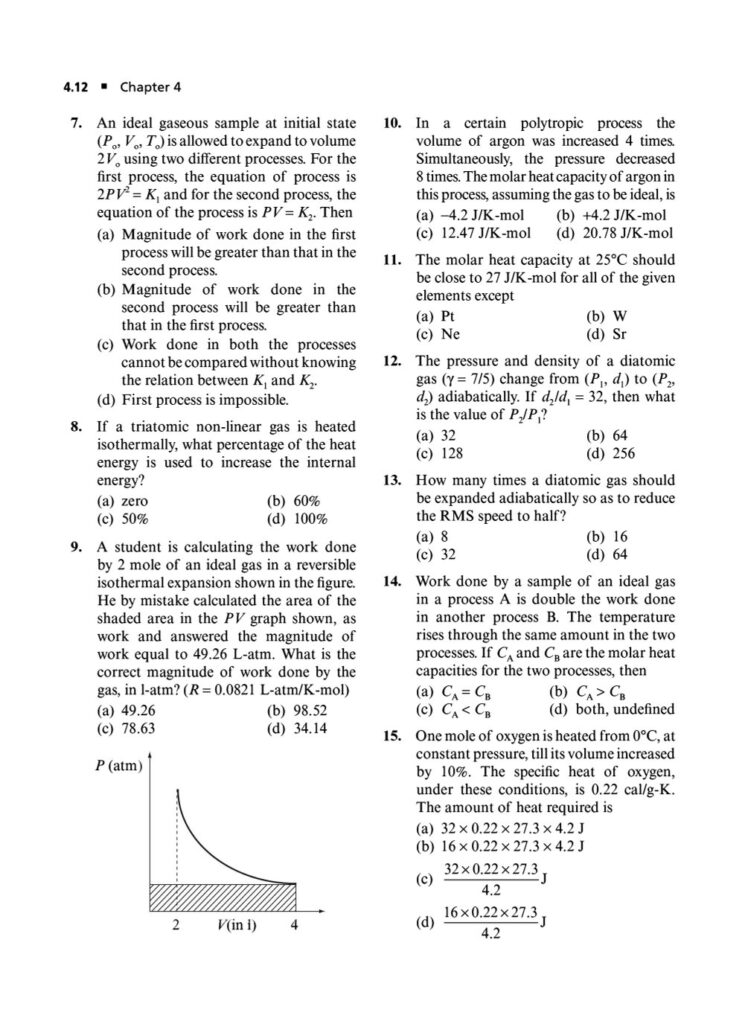
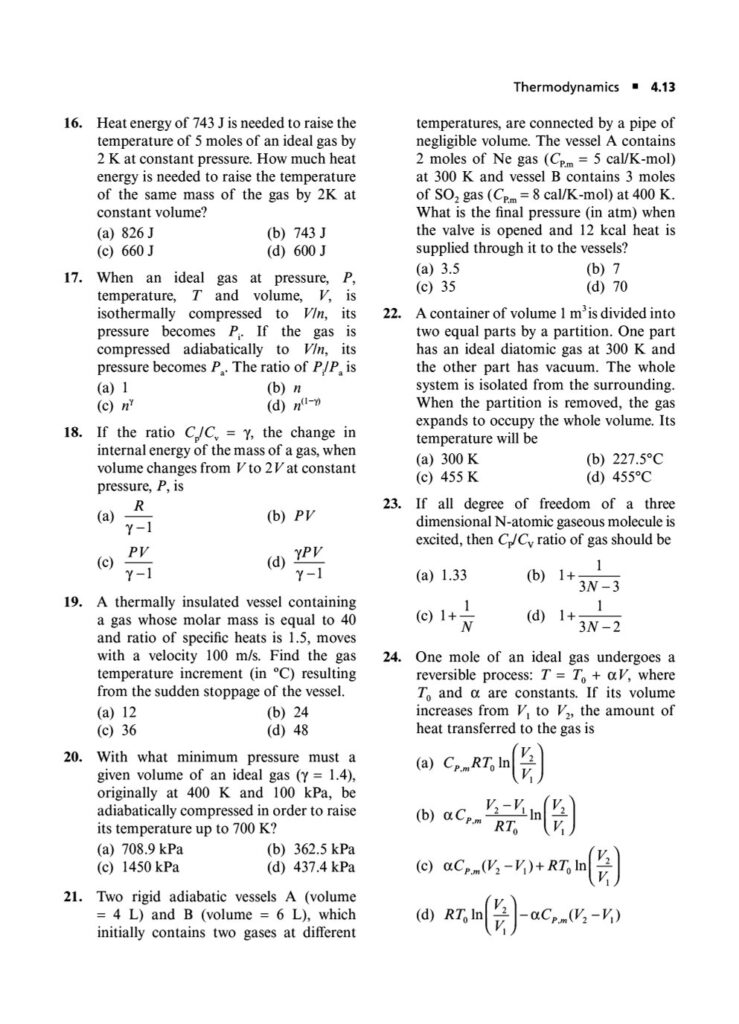
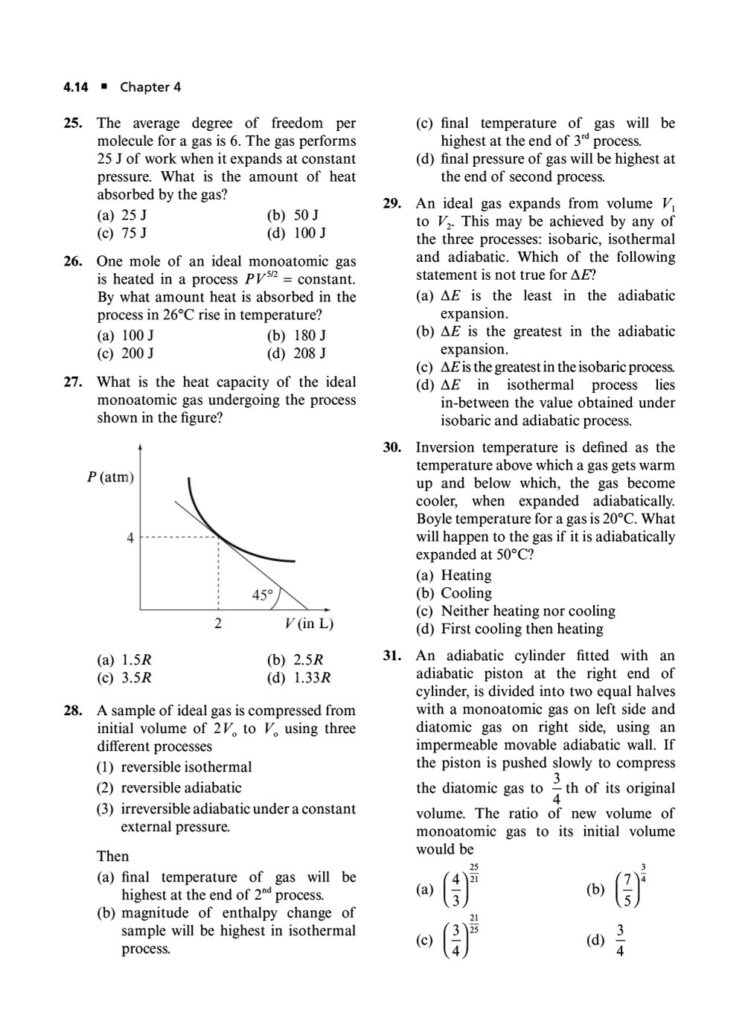
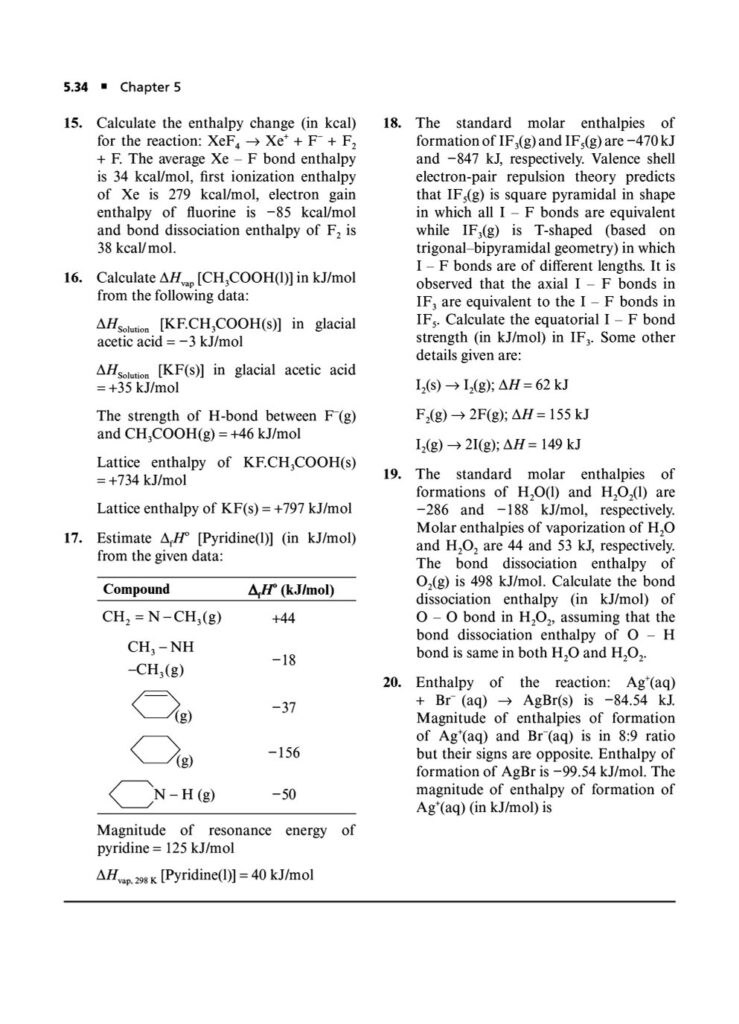
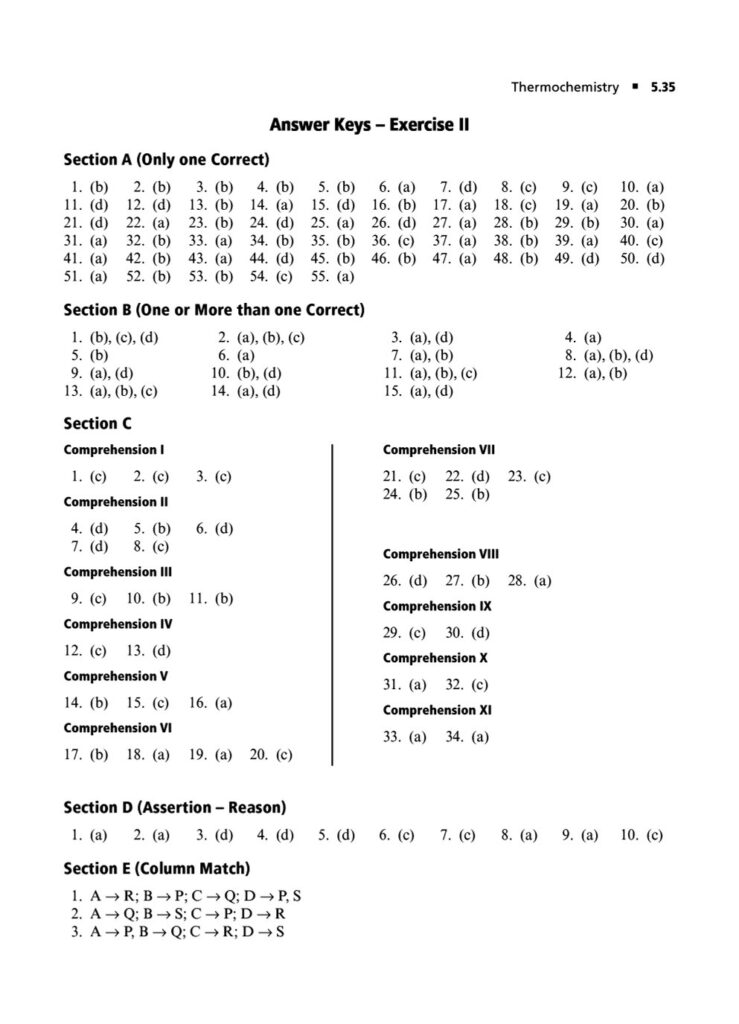
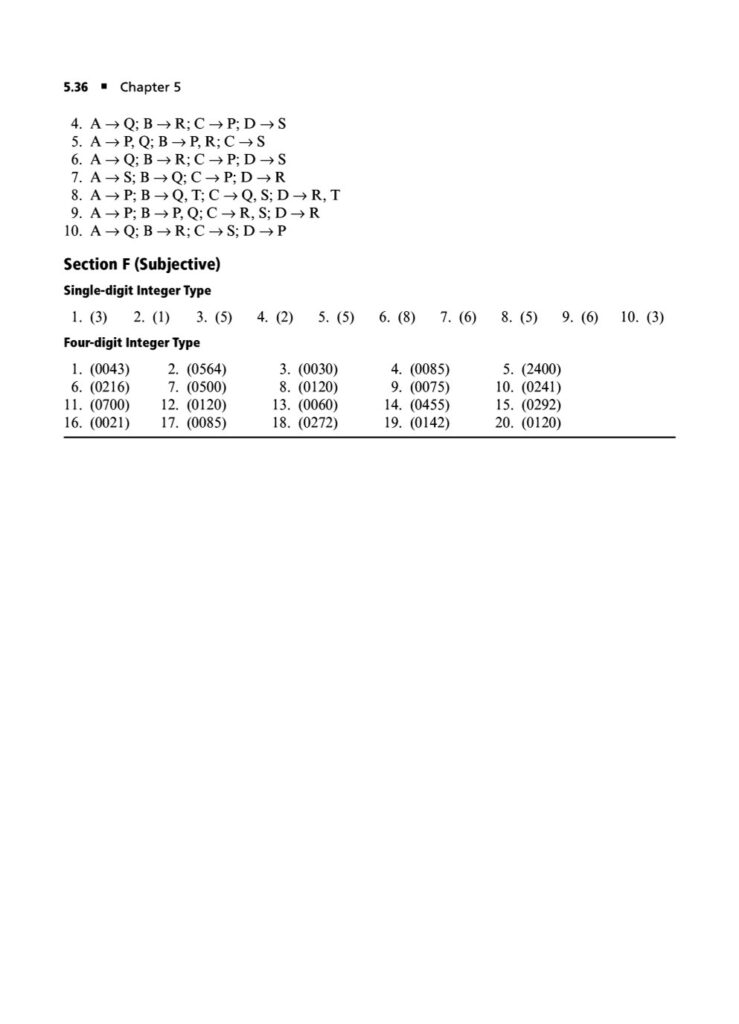
In the world of JEE Advanced, a profound understanding of thermodynamics is essential. It’s not enough to simply know the formulas; you must possess the conceptual depth and analytical rigor to apply them to intricate, multi-step problems. Thermodynamics in JEE Advanced often involves combining concepts, interpreting graphs, and dealing with complex systems, making it a critical topic for distinguishing top performers.
In this section, we’ll delve into the world of thermodynamics with a focus on JEE Advanced-level questions. We’ll explore the deeper applications of the fundamental principles, tackling problems that require a synthesis of knowledge from various areas. The questions are designed to challenge your understanding, build your problem-solving skills, and prepare you for the unique demands of the JEE Advanced exam.
Our thermodynamics JEE Advanced questions will cover topics such as:
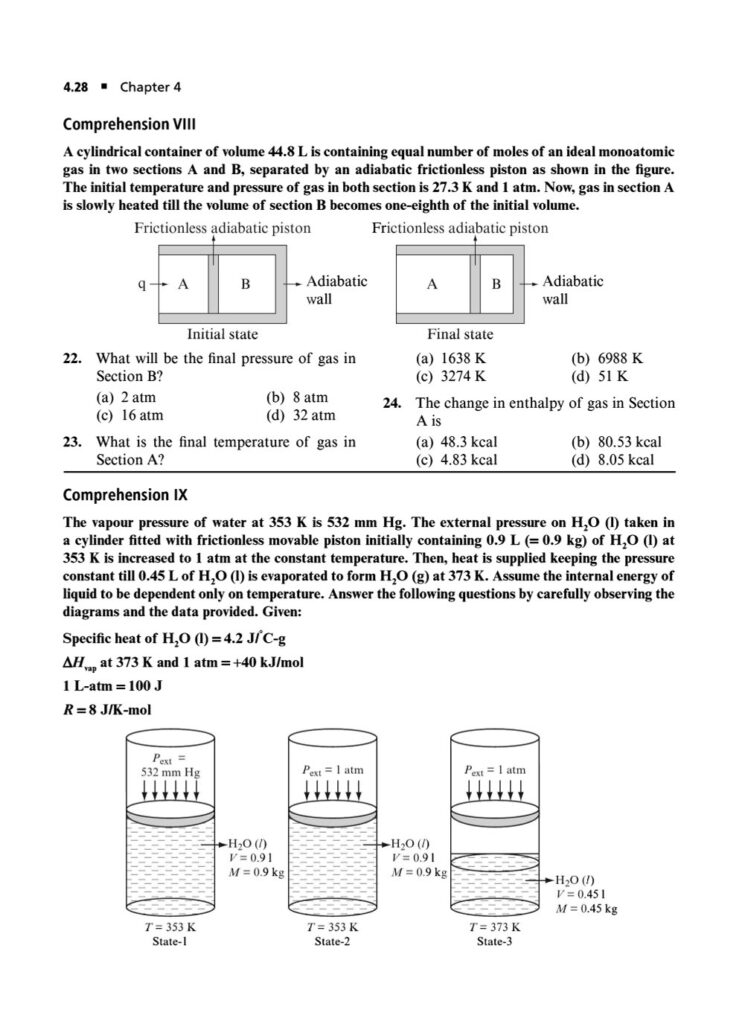
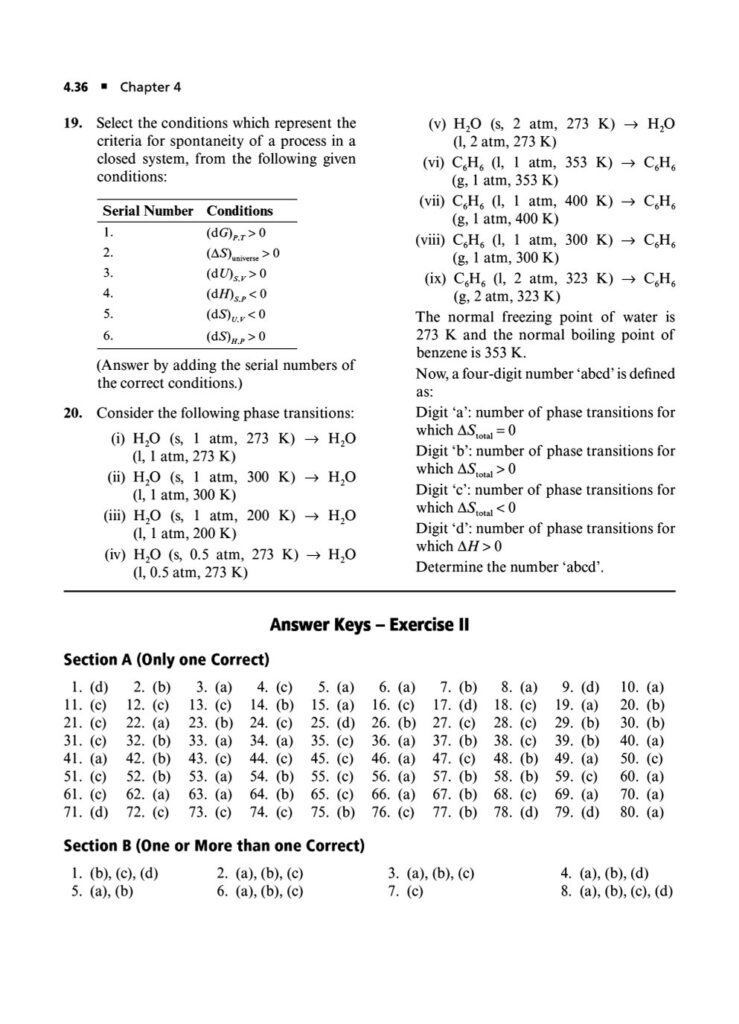
Phase Transitions: Calculating thermodynamic quantities like ΔH and ΔS for changes of state (melting, boiling, sublimation).ing you to systematically strengthen your grasp on the principles of chemical and ionic equilibrium.
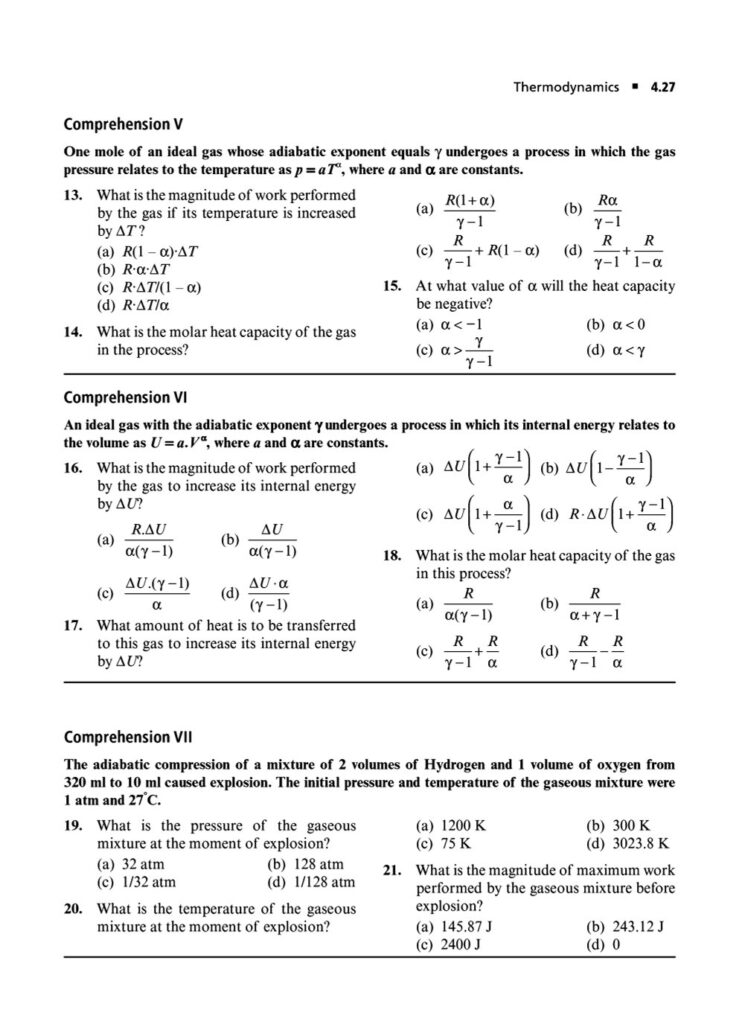
First Law of Thermodynamics: In-depth application of ΔU=q+w for various processes, including reversible and irreversible expansions/compressions, and graphical analysis of P-V diagrams.
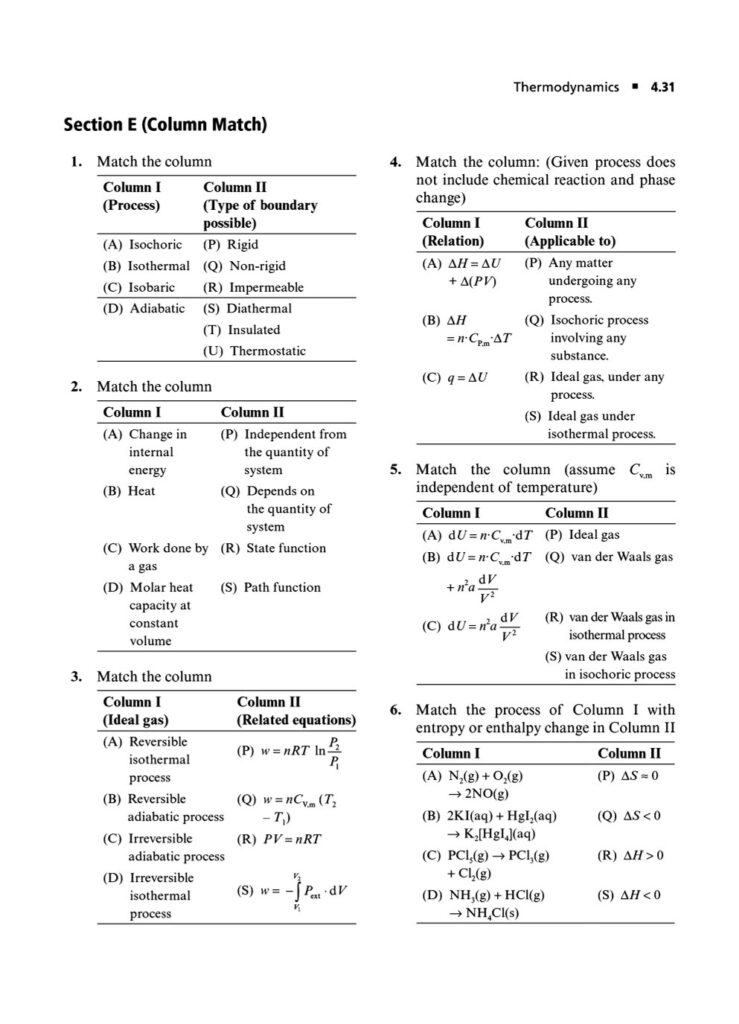
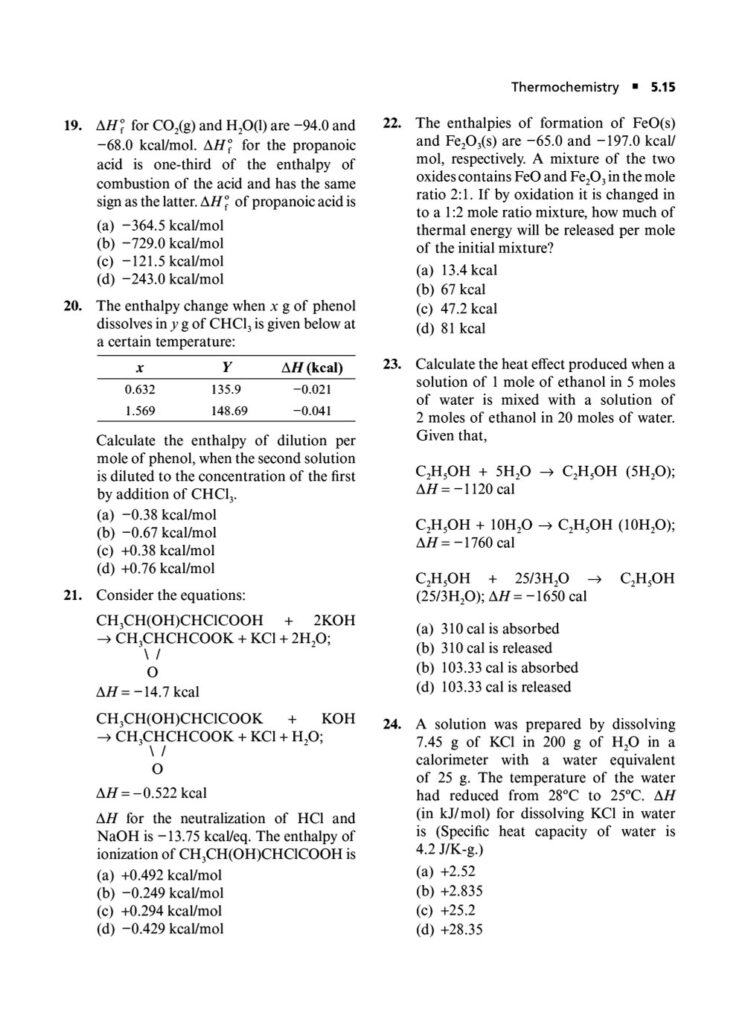
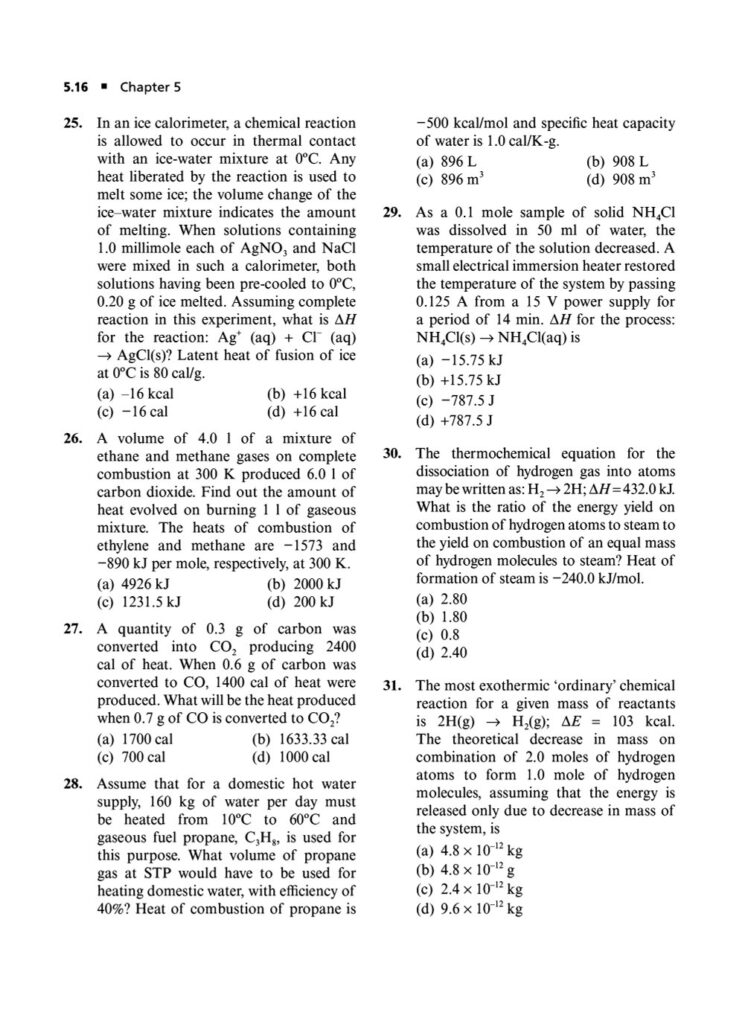
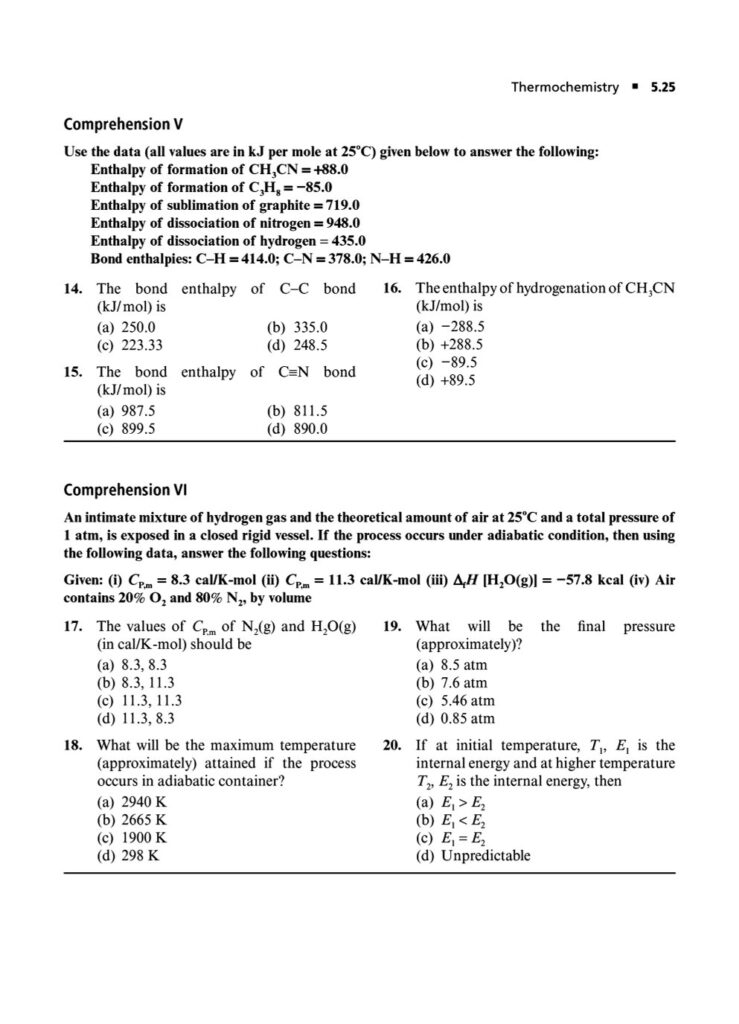
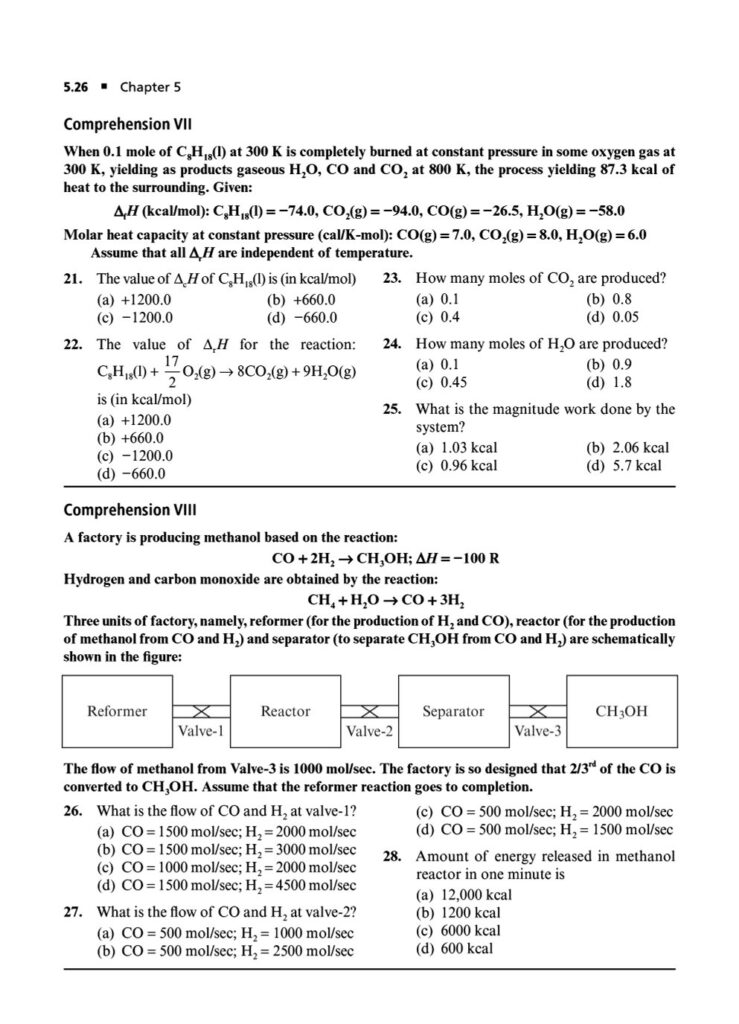
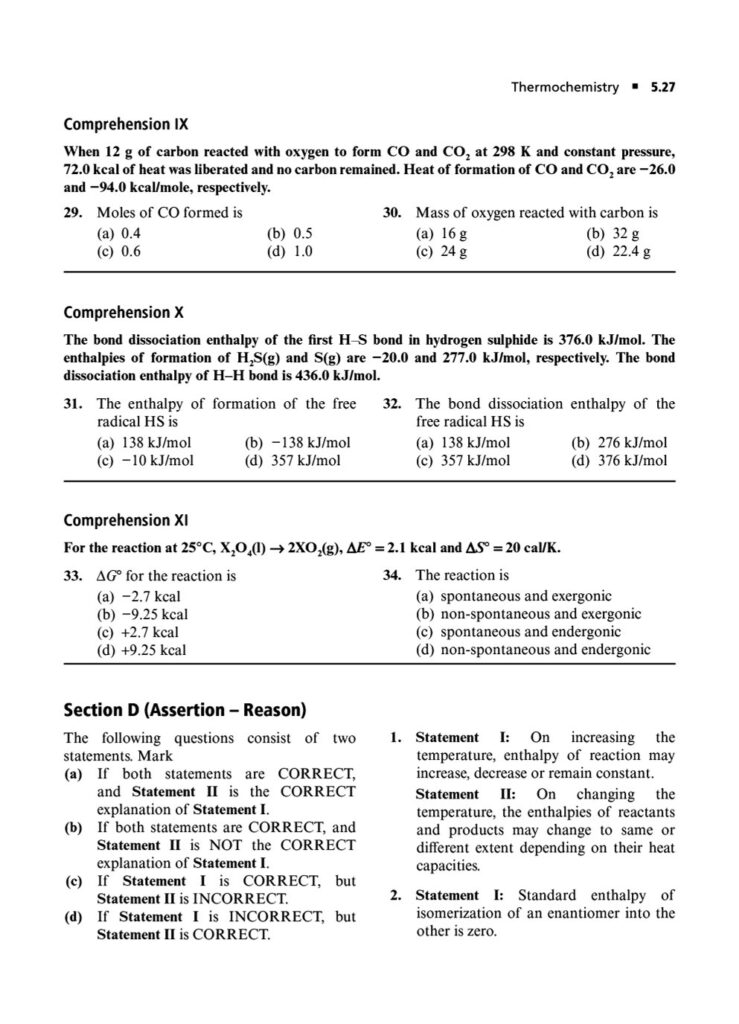
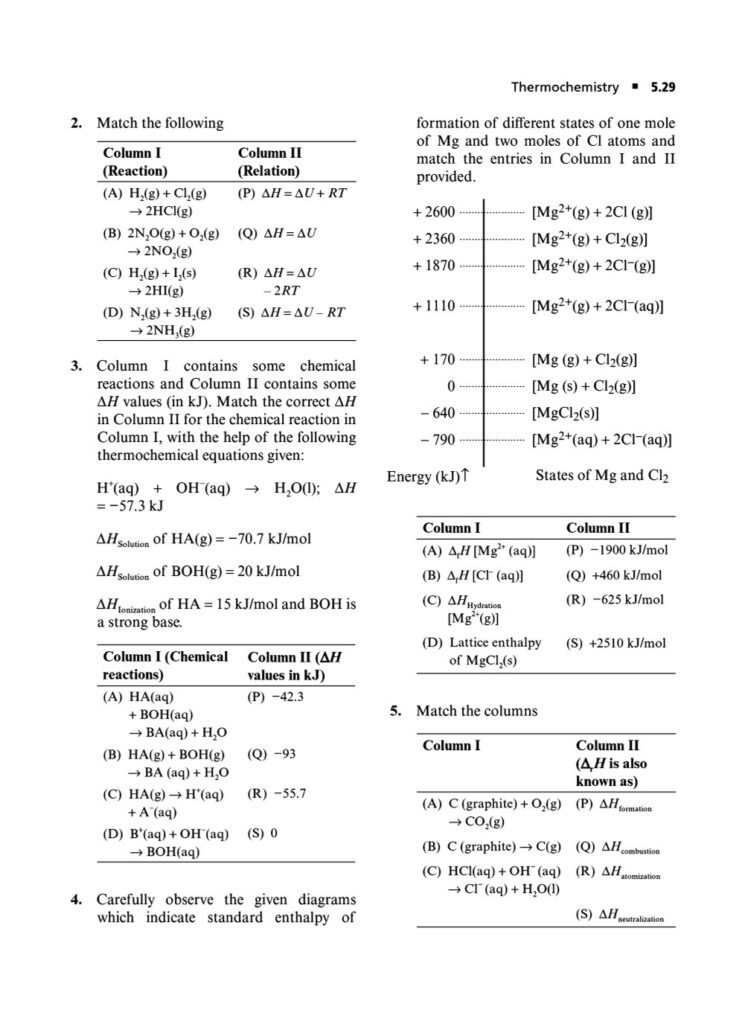
Enthalpy, Heat Capacity, and Thermochemistry: Advanced problems involving Hess’s Law, calculating bond energies, and the temperature dependence of enthalpy.
Second Law of Thermodynamics: Calculating entropy changes (ΔS) for physical and chemical processes, including phase transitions and mixing of gases.
Gibbs Free Energy and Spontaneity: The relationship between ΔG, ΔH, ΔS, and the equilibrium constant (Keq). Solving problems using the Gibbs-Helmholtz equation.
Thermodynamic cycles: Analyzing thermodynamic processes in cycles and calculating efficiency and net work done.
Multi-concept Problems: Questions that integrate thermodynamics with other topics like chemical equilibrium, electrochemistry, and physical properties of gases and liquids.
Non-ideal Gases: Applying thermodynamic principles to real gases and van der Waals gases, where the ideal gas assumptions do not hold.