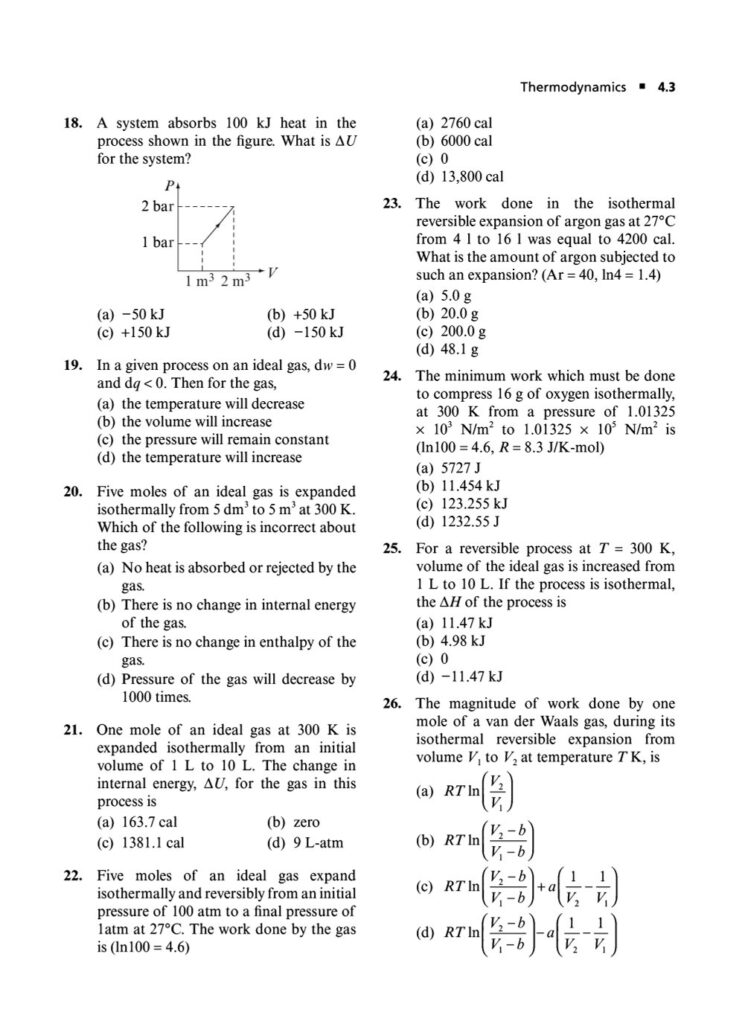
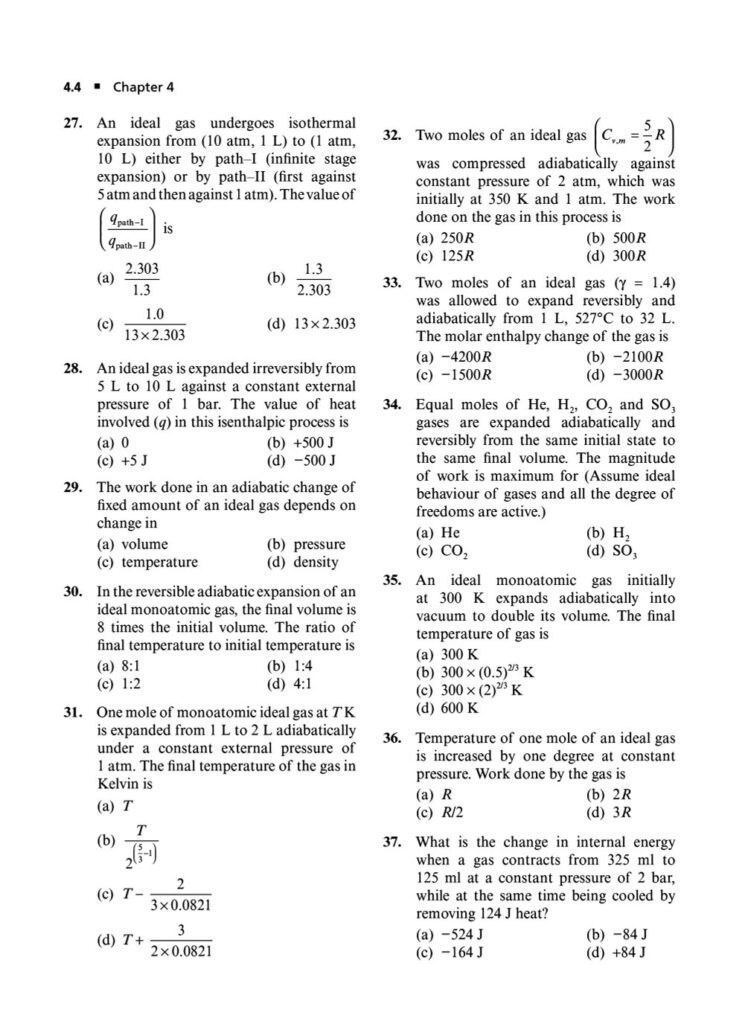
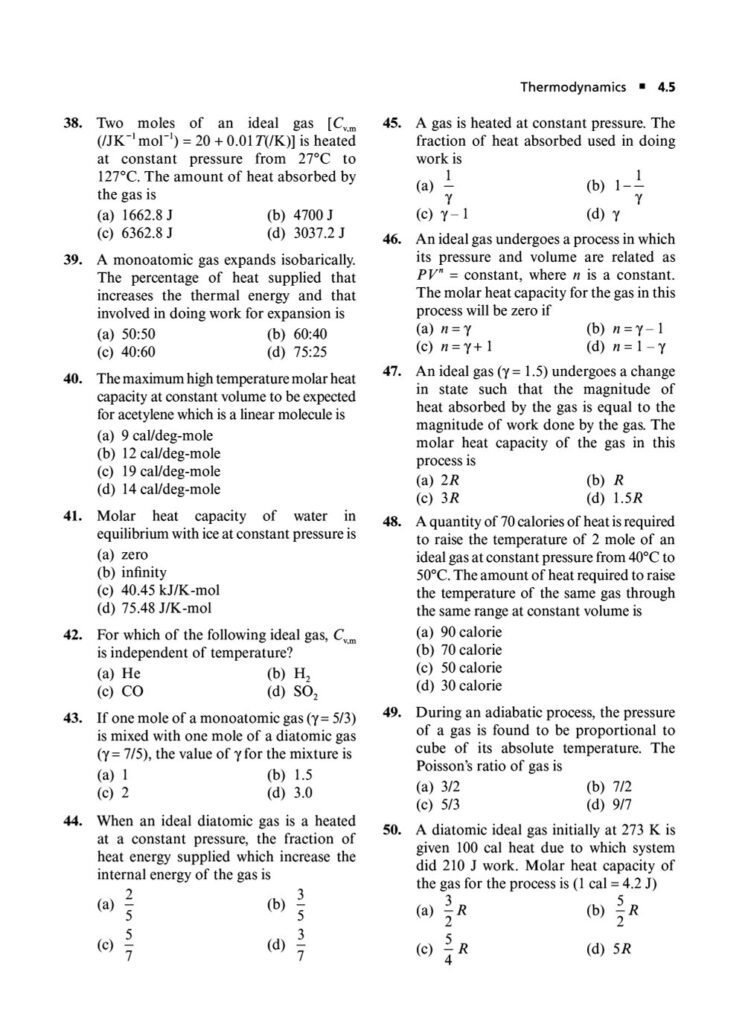
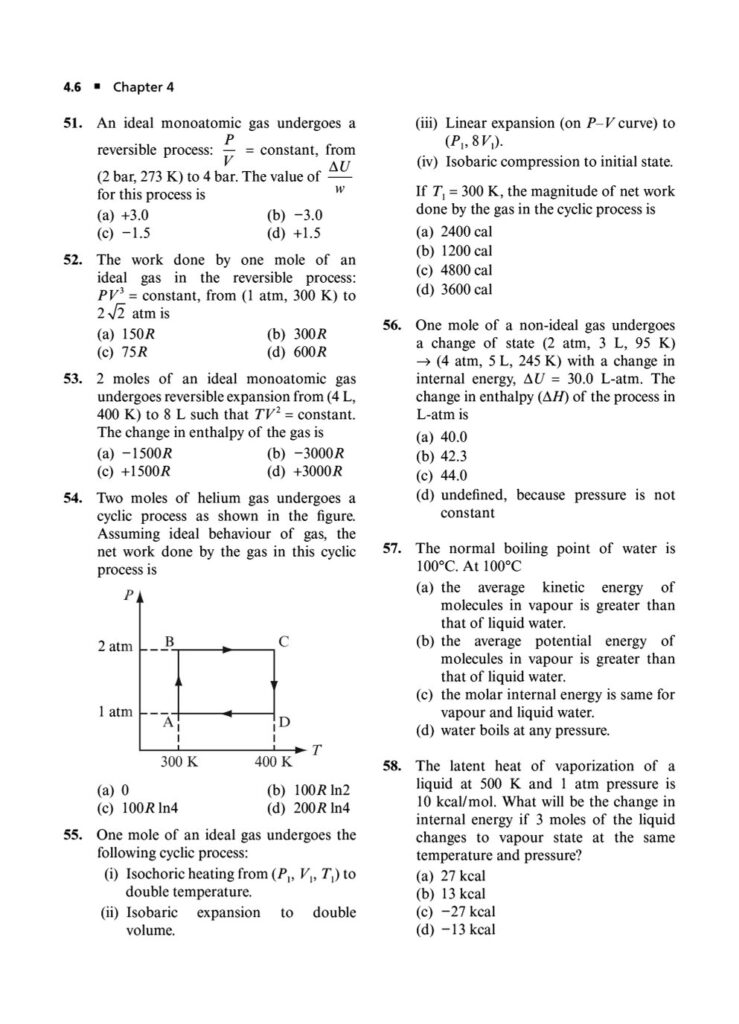
In the world of chemistry and physics, thermodynamics is the study of energy, its transformations, and its relationship with matter. It’s a powerful and fundamental concept that explains everything from the efficiency of engines to the spontaneity of chemical reactions. Mastering thermodynamics is crucial for cracking the Joint Entrance Examination (JEE) Main.
In this section, we’ll dive into the world of thermodynamics, exploring the principles, laws, and formulas that govern these energy transformations. We’ll tackle a range of questions, from basic to advanced, to help you build a strong foundation and boost your confidence in tackling thermodynamics problems in the JEE Main exam.
Our thermodynamics JEE Main questions will cover topics such as:
- First Law of Thermodynamics: The relationship between internal energy (ΔU), heat (q), and work (w), expressed as ΔU=q+w.
- Enthalpy (ΔH): The heat absorbed or released during a reaction at constant pressure.
- Work Done in various processes: Calculations for isothermal, adiabatic, isobaric, and isochoric processes.
- Second Law of Thermodynamics and Entropy (ΔS): The concept of spontaneity and the measure of a system’s disorder.
- Third Law of Thermodynamics: The entropy of a perfect crystal at absolute zero.
- Gibbs Free Energy (ΔG): The criterion for spontaneity, expressed by the equation ΔG=ΔH−TΔS.
- Heat Capacity (Cp and Cv): The amount of heat required to change the temperature of a substance.
- Thermodynamic Processes: Understanding and applying the principles of reversible and irreversible processes.
- Hess’s Law and Born-Haber Cycle: Calculating enthalpy changes for reactions.