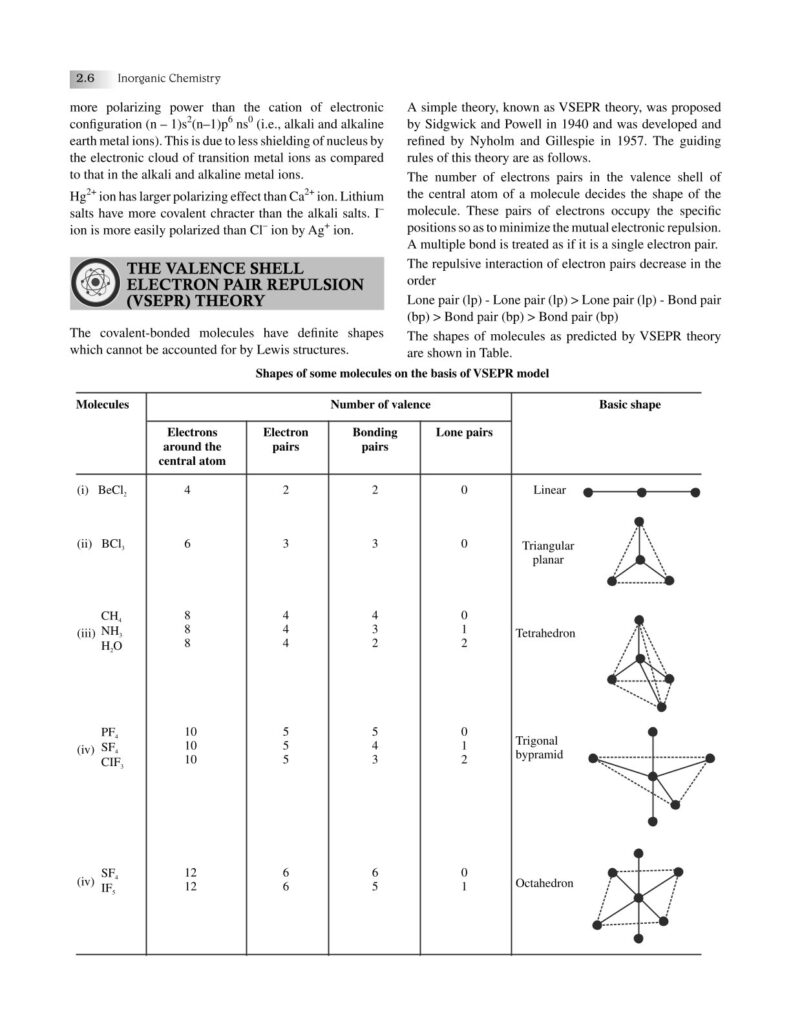
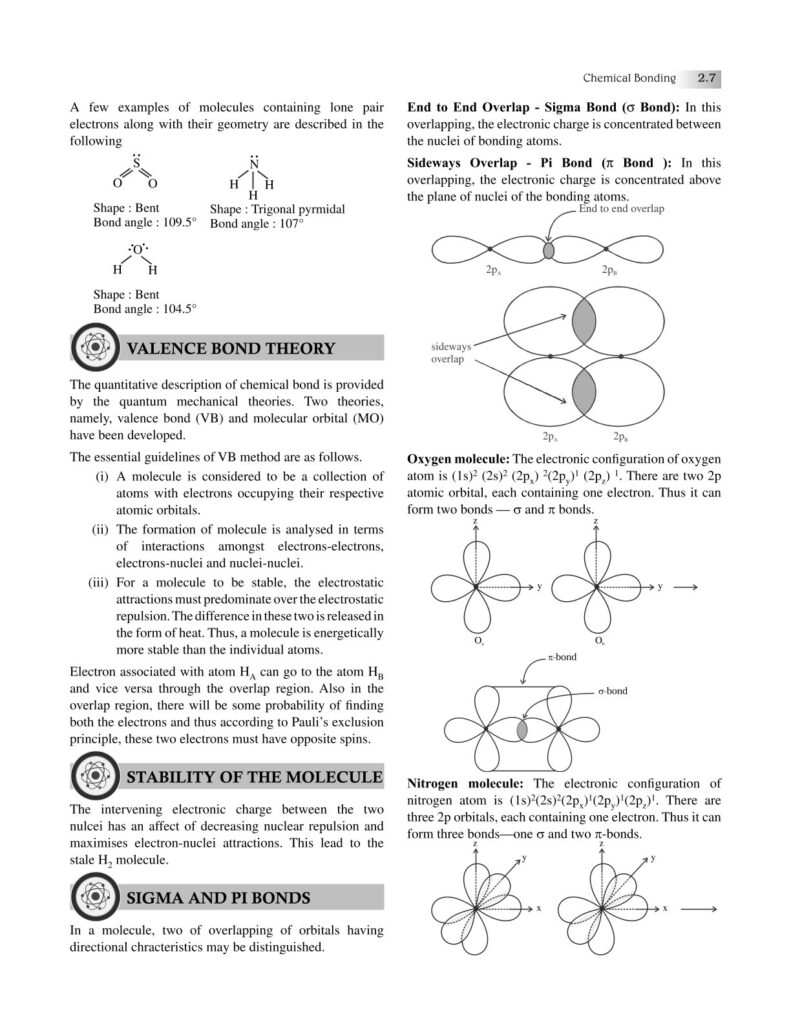
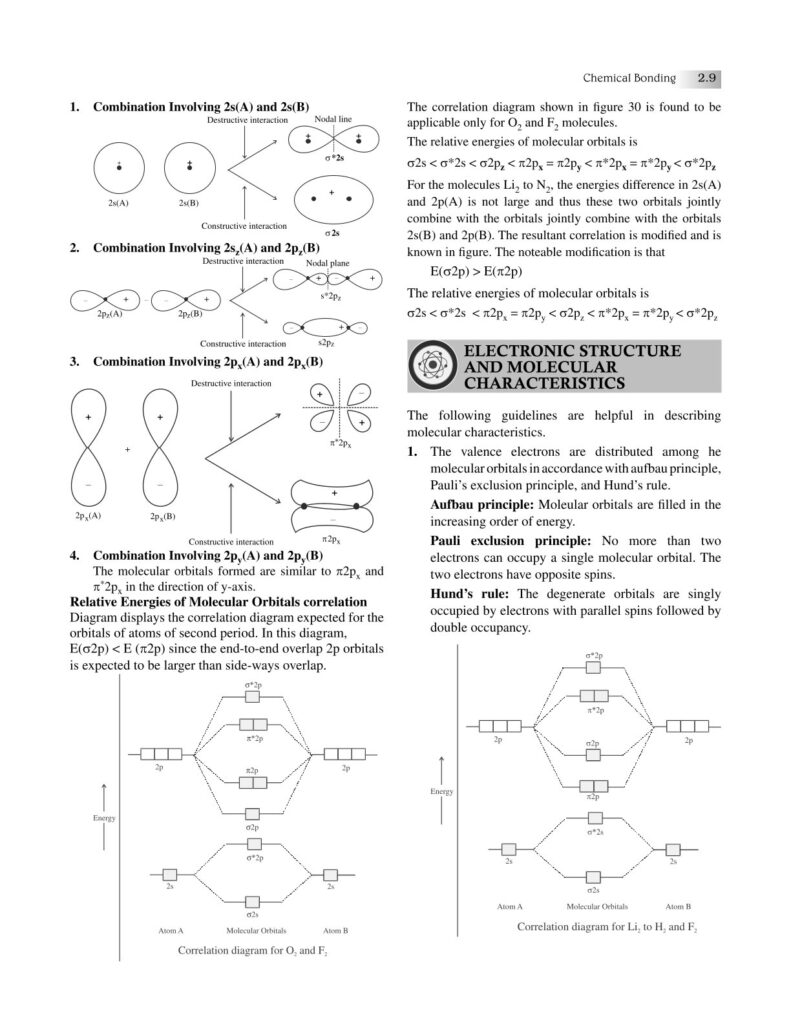
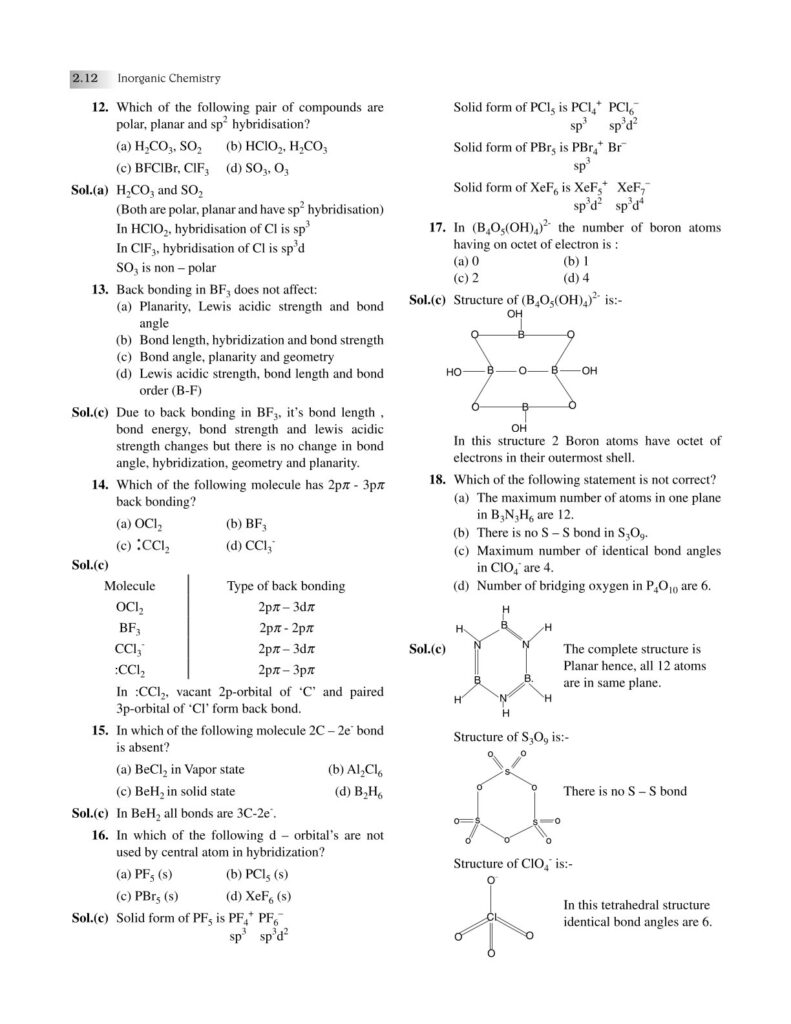
Chemical bonding is one of the most fundamental concepts in chemistry, forming the very basis of how elements combine to create the diverse substances that make up our universe. From the air we breathe to the food we eat, from the medicines that heal us to the materials that build our world—everything is held together by bonds at the atomic level. Understanding chemical bonding allows us to decode the stability, structure, and properties of matter, bridging the gap between microscopic atomic interactions and the macroscopic world we observe.
In Class 11 Chemistry, the topic of chemical bonding explores why atoms interact, how they share or transfer electrons, and the forces that keep them bound together. This chapter introduces key theories and models—such as the Octet Rule, Valence Bond Theory, Hybridisation, and Molecular Orbital Theory—that help explain the shape, polarity, and strength of molecules. It also covers the major types of bonds like ionic, covalent, and metallic bonds, along with concepts like bond parameters, VSEPR theory, and hydrogen bonding.
A strong grasp of chemical bonding not only strengthens your foundation for advanced chemistry topics in Class 12 and competitive exams but also enhances your logical and visualisation skills in understanding molecular structures. As you delve deeper, you’ll realise that chemical bonding is not just a chapter—it’s the language through which chemistry speaks.